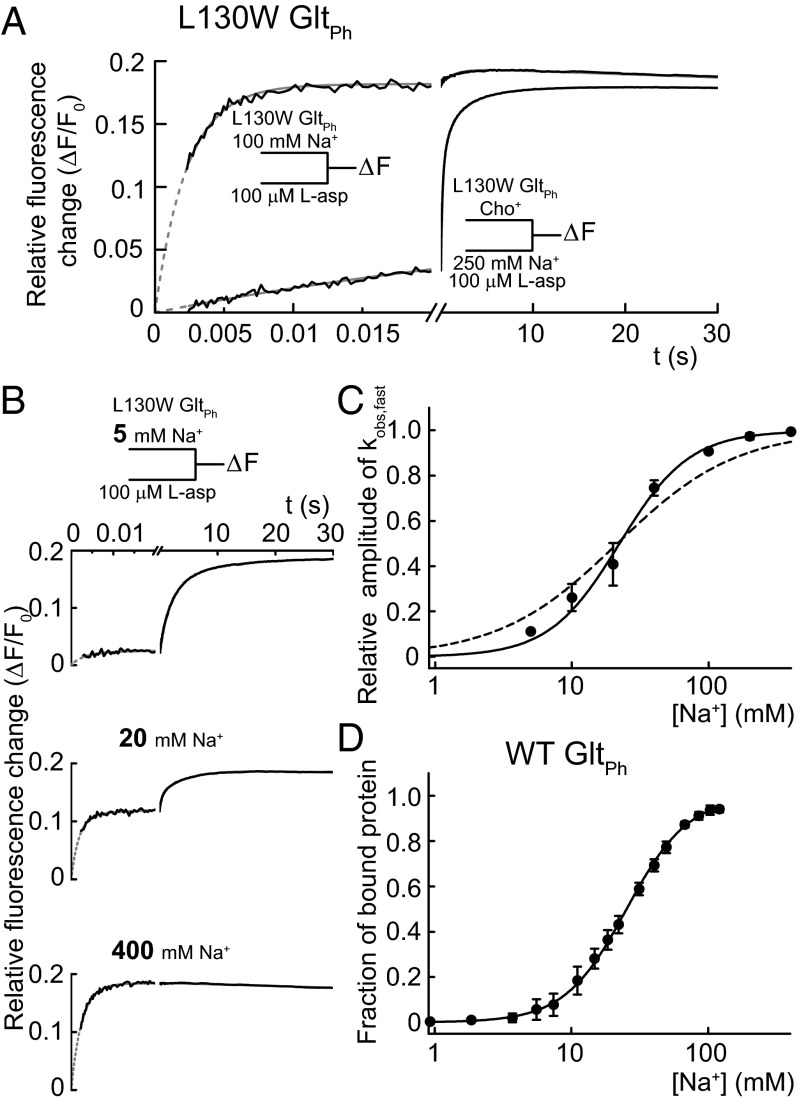
Fig. 2.
Fast aspartate binding after slow processes associated with the binding of two Na+. (A) The tryptophan fluorescence transients upon the rapid addition of 100 µM l-aspartate to L130W GltPh preequilibrated with 100 mM NaCl (upper curve) or mixing 100 µM l-aspartate and 250 mM NaCl with empty L130W GltPh (lower curve). NaCl (500 mM) was equimolarly substituted with choline chloride. The black lines represent experimental data, and the fits to the sums of exponential functions as well as a linear function to account for photobleaching are in gray. (B) Fluorescence transients and corresponding exponential fits after mixing 100 µM l-aspartate with L130W GltPh preincubated with three different [Na+] (the fit results of all experiments are in Table S1). (C) The relative amplitudes of the highest rates obtained from the fits (kobs,fast) as a function of [Na+]. The continuous line represents the fit to a Hill function with nHill = 1.7 ± 0.3 and KD = 22 ± 3 mM. For comparison, the result of a fit with nHill fixed to one is shown as a broken line. (D) The Na+ titration curve of WT GltPh emission at 308 nm after excitation at 289 nm in 500 mM choline chloride fitted with a Hill function (nHill = 1.9 ± 0.2, KD = 25 ± 2 mM) is shown as a continuous line. The fraction of bound protein is calculated by dividing the relative fluorescence change caused by each addition by the maximum change. All error bars represent ±SEM of three experiments.