Abstract
Cobalamin (Cbl; vitamin B12) malabsorption in pancreatic insufficiency can be partially corrected by bicarbonate and completely corrected by pancreatic proteases but the mechanisms involved are unknown. Because saliva contains enough R-type Cbl-binding protein (R protein) to bind all of the dietary and biliary Cbl, it is possible that R protein acts as an inhibitor of Cbl absorption and that pancreatic proteases are required to alter R protein and prevent such inhibition. To test this hypothesis we studied the ability of R protein and intrinsic factor (IF) to compete for Cbl binding and ability of pancreatic proteases to alter this competition.
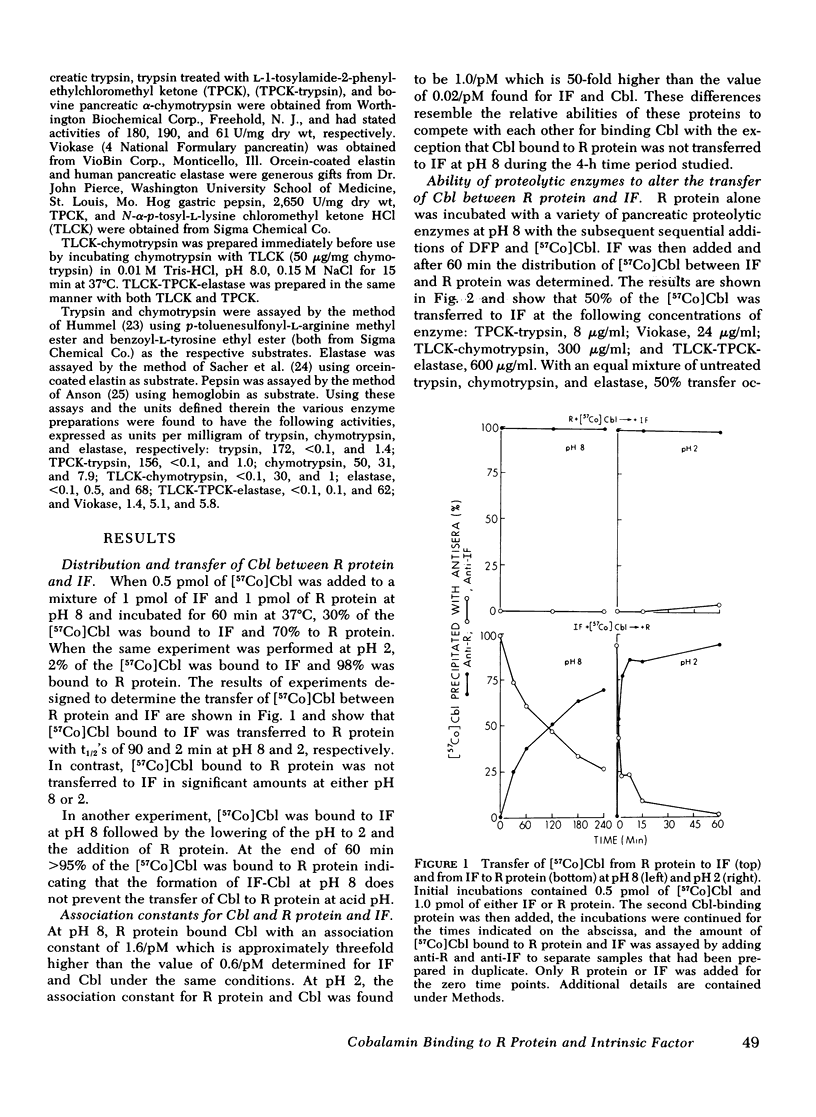
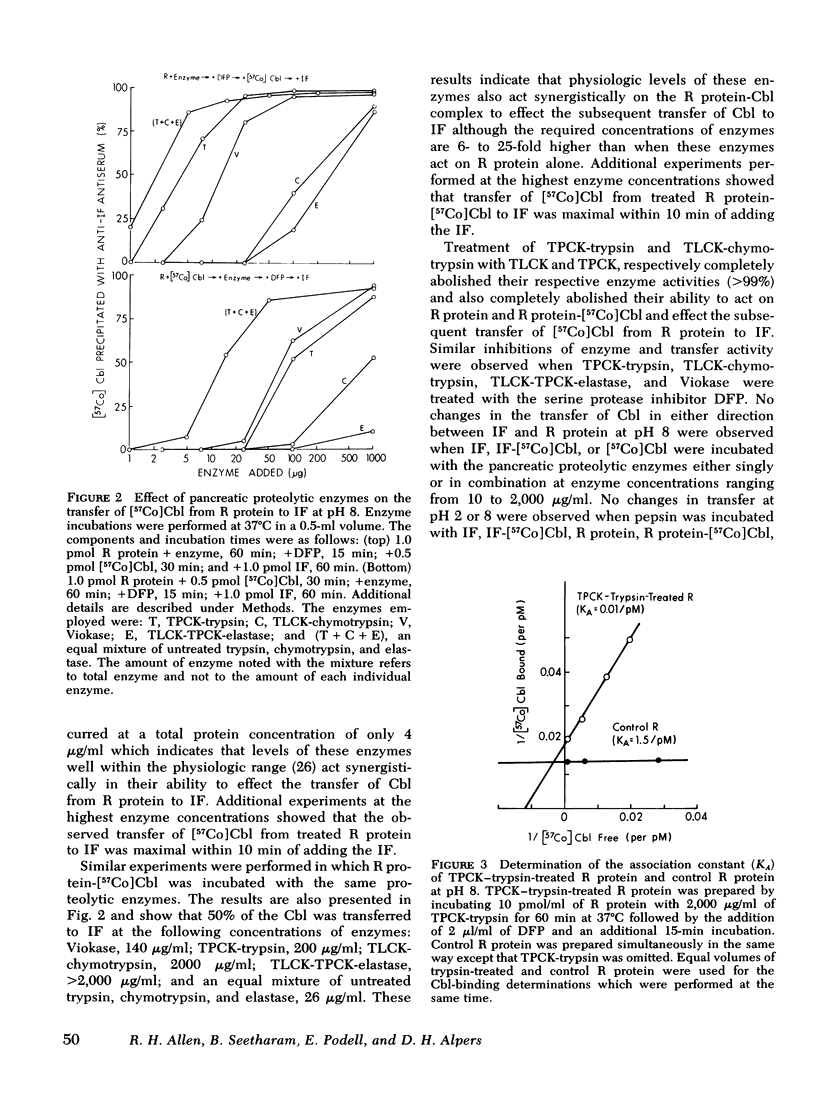
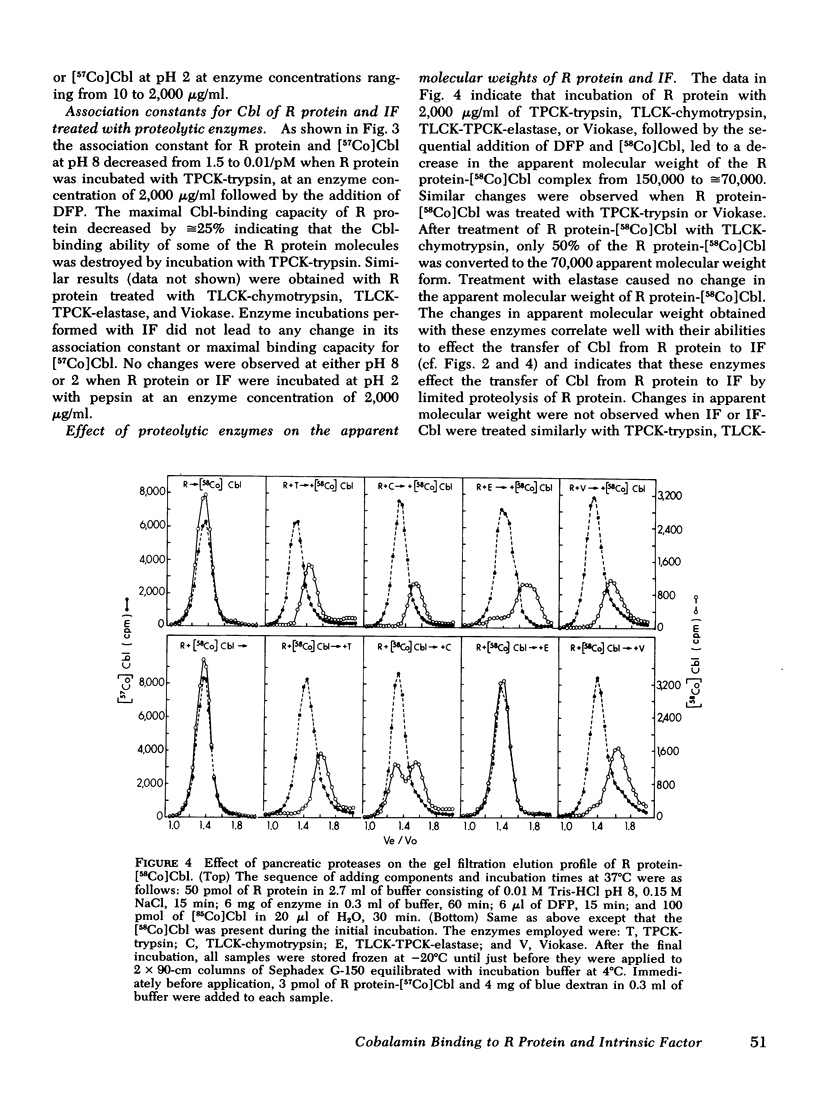
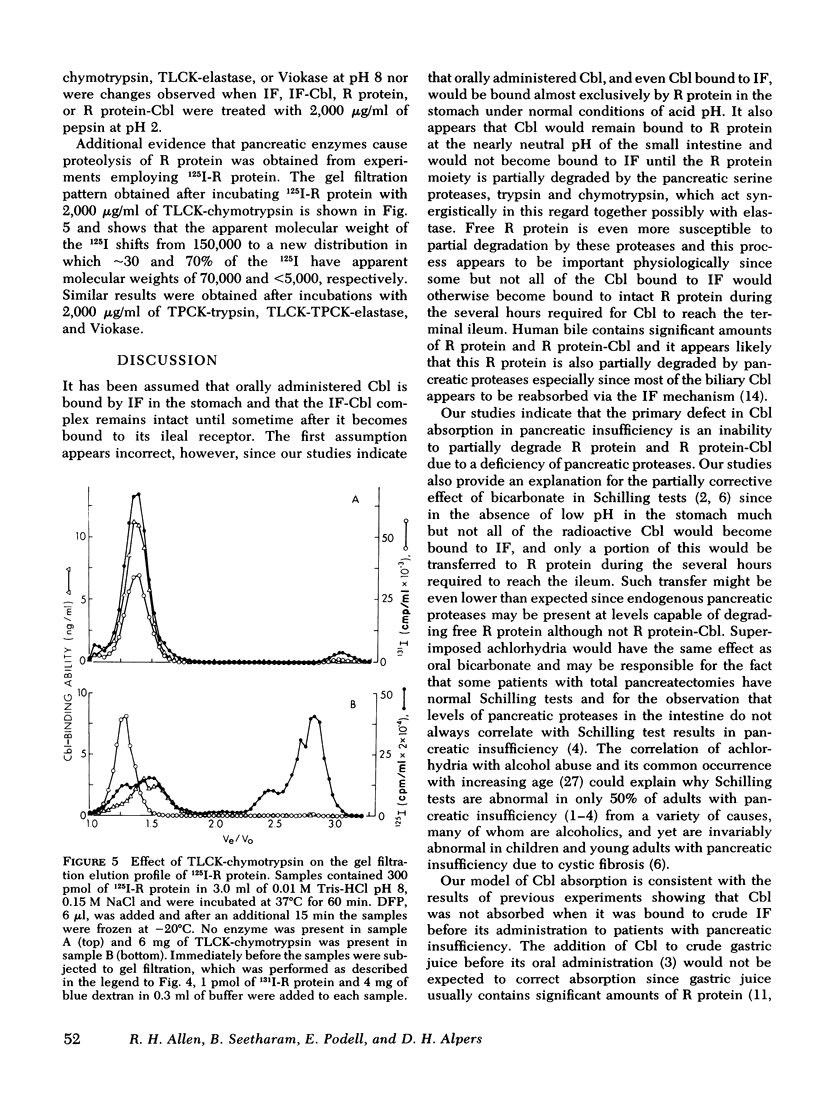
Human salivary R protein bound Cbl with affinities that were 50- and 3-fold higher than those of human IF at pH 2 and 8, respectively. Cbl bound to IF was transferred to an equal amount of R protein with t½'s of 2 and 90 min at pH 2 and 8, respectively, and within several hours respective ratios of R protein-Cbl/IF-Cbl of 50 and 2 were observed. Cbl bound to R protein was not transferred to IF at either pH 2 or 8. Incubation of R protein with pancreatic proteases at pH 8 led to a 150-fold decrease in its affinity for Cbl. Incubation of R protein-Cbl with pancreatic proteases led to complete transfer of Cbl to IF within 10 min. Gel filtration studies with R protein-[57Co]Cbl and 125I-R protein showed that pancreatic proteases partially degraded R protein. Pancreatic proteases differed in their ability to effect these changes with trypsin > chymotrypsin > elastase. Pancreatic proteases did not alter IF in any of the parameters mentioned above. Pepsin failed to alter either R protein or IF.
These studies suggest the following: (a) that Cbl is bound almost exclusively to R protein in the acid milieu of the stomach, rather than to IF as has been assumed previously; (b) that Cbl remains bound to R protein in the slightly alkaline environment of the intestine until pancreatic proteases partially degrade R protein and enable Cbl to become bound exclusively to IF; and (c) that the primary defect in Cbl absorption in pancreatic insufficiency is a lack of pancreatic proteases and a failure to alter R protein and effect the transfer of Cbl to IF. These studies also suggest that the partial correction of Cbl malabsorption observed with bicarbonate is due to neutralization of gastric HCl, since at slightly alkaline, pH IF can partially compete with R protein for the initial binding and retention of Cbl.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H. Human vitamin B12 transport proteins. Prog Hematol. 1975;9:57–84. [PubMed] [Google Scholar]
- Allen R. H., Mehlman C. S. Isolation of gastric vitamin B 12 -binding proteins using affinity chromatography. I. Purification and properties of human intrinsic factor. J Biol Chem. 1973 May 25;248(10):3660–3669. [PubMed] [Google Scholar]
- Allen R. H., Mehlman C. S. Isolation of gastric vitamin B 12 -binding proteins using affinity chromatography. II. Purification and properties of hog intrinsic factor and hog nonintrinsic factor. J Biol Chem. 1973 May 25;248(10):3670–3680. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. L., Allen R. H. Characterization of vitamin B12-binding proteins isolated from human milk and saliva by affinity chromatography. J Biol Chem. 1974 Nov 25;249(22):7220–7227. [PubMed] [Google Scholar]
- CONLEY C. L., KREVANS J. R., MCINTYRE P. A., SACHS M. V. Pathogenesis and treatment of macrocytic anemia; information obtained with radioactive vitamin B12. AMA Arch Intern Med. 1956 Nov;98(5):541–549. doi: 10.1001/archinte.1956.00250290001001. [DOI] [PubMed] [Google Scholar]
- Deren J. J., Arora B., Toskes P. P., Hansell J., Sibinga M. S. Malabsorption of crystalline vitamin B 12 in cystic fibrosis. N Engl J Med. 1973 May 3;288(18):949–950. doi: 10.1056/NEJM197305032881808. [DOI] [PubMed] [Google Scholar]
- Gräsbeck R., Salonen E. M. Vitamin B12. Prog Food Nutr Sci. 1976;2(5):193–231. [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Katz M., Mehlman C. S., Allen R. H. Isolation and characterization of an abnormal human intrinsic factor. J Clin Invest. 1974 May;53(5):1274–1283. doi: 10.1172/JCI107674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977 Dec;60(6):1381–1392. doi: 10.1172/JCI108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuchansky C., Rambaud J. C., Modigliani R., Bernier J. J. Letter: Vitamin B12 malabsorption in chronic pancreatitis. Gastroenterology. 1974 Aug;67(2):406–407. [PubMed] [Google Scholar]
- Okuda K., Kitazaki T., Takamatsu M. Inactivation of vitamin B12 by a binder in rat intestine and the role of intrinsic factor. Digestion. 1971;4(1):35–48. doi: 10.1159/000197094. [DOI] [PubMed] [Google Scholar]
- SACHAR L. A., WINTER K. K., SICHER N., FRANKEL S. Photometric method for estimation of elastase activity. Proc Soc Exp Biol Med. 1955 Nov;90(2):323–326. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Burger R. L., Mehlman C. S., Allen R. H. The role and fate of rabbit and human transcobalamin II in the plasma transport of vitamin B12 in the rabbit. J Clin Invest. 1976 Jan;57(1):27–38. doi: 10.1172/JCI108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toskes P. P., Deren J. J., Fruiterman J., Conrad M. E. Specificity of the correction of vitamin B12 malabsorption by pancreatic extract and its clinical significance. Gastroenterology. 1973 Aug;65(2):199–204. [PubMed] [Google Scholar]
- Toskes P. P., Deren J. J. The role of the pancreas in vitamin B 12 absorption: studies of vitamin B 12 absorption in partially pancreatectomized rats. J Clin Invest. 1972 Feb;51(2):216–223. doi: 10.1172/JCI106806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toskes P. P., Deren J. J. Vitamin B 12 absorption and malabsorption. Gastroenterology. 1973 Oct;65(4):662–683. [PubMed] [Google Scholar]
- Toskes P. P., Hansell J., Cerda J., Deren J. J. Vitamin B 12 malabsorption in chronic pancreatic insufficiency. N Engl J Med. 1971 Mar 25;284(12):627–632. doi: 10.1056/NEJM197103252841202. [DOI] [PubMed] [Google Scholar]
- Toskes P. P., Smith G. W., Francis G. M., Sander E. G. Evidence that pancreatic proteases enhance vitamin B12 absorption by acting on curde preparations of hog gastric intrinsic factor and human gastric juice. Gastroenterology. 1977 Jan;72(1):31–36. [PubMed] [Google Scholar]
- Von der Lippe G., Andersen K. J., Schjönsby H. Pancreatic extract and the intestinal uptake of vitamin B12. III. Stimulatory effect in the presence of a non-intrinsic factor vitamin B12 binder. Scand J Gastroenterol. 1977;12(2):183–187. [PubMed] [Google Scholar]


