Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants and are carcinogenic in multiple organs and species. Benzo[a]pyrene (B[a]P) is a representative PAH and has been studied extensively for its carcinogenicity and toxicity. B[a]P itself is chemically inert and requires metabolic activation to exhibit its toxicity and carcinogenicity. Three major metabolic pathways have been well documented. The signature metabolites generated from the radical cation (peroxidase or monooxygenase mediated) pathway are B[a]P-1,6-dione and B[a]P-3,6-dione, the signature metabolite generated from the diol-epoxide (P450 mediated) pathway is B[a]P-r-7,t-8,t-9,c-10-tetrahydrotetrol (B[a]P-tetrol-1) and the signature metabolite generated from the o-quinone (aldo-keto reductase mediated) pathway is B[a]P-7,8-dione. The contributions of these different metabolic pathways to cancer initiation and the exploitation of this information for cancer prevention are still under debate. With the availability of a library of [13C4]-labeled B[a]P metabolite internal standards, we developed a sensitive stable isotope dilution atmospheric pressure chemical ionization tandem mass spectrometry method to address this issue by quantitating B[a]P metabolites from each metabolic pathway in human lung cells. This analytical method represents a 500 fold increased sensitivity compared with a method using HPLC-radiometric detection. The limit of quantitation (LOQ) was determined to be 6 fmol on column for 3-hydroxybenzo[a]pyrene (3-OH-B[a]P), the generally accepted biomarker for B[a]P exposure. This high level of sensitivity and robustness of the method was demonstrated in a study of B[a]P metabolic profiles in human bronchoalveolar H358 cells induced or uninduced with the AhR ligand, 2,3,7,8-tetrachlorodibenzodioxin (TCDD). All the signature metabolites were detected and successfully quantitated. Our results suggest that all three metabolic pathways contribute equally in the overall metabolism of B[a]P in H358 cells with or without TCDD induction. The sensitivity of the method should permit the identification of cell-type differences in B[a]P activation and detoxication and could also be used for biomonitoring human exposure to PAH.
Keywords: aldo-keto reductase, benzo[a]pyrene, H358 cell, metabolome, P450 enzymes, stable isotope dilution mass spectrometry, TCDD induction
Introduction
PAHs are a unique class of compounds characterized by two or more fused aromatic rings and contain no heteroatoms. They are products of fossil fuel combustion, and are found in cigarette smoke, automotive exhaust and smoked and charcoal broiled food and are one of the most ubiquitous environmental pollutants.1 Humans are exposed to PAHs from various sources such as inhalation through the lung, ingestion from food, and absorption through the skin. PAH mixtures and individual PAHs are known to be carcinogenic and/or co-carcinogenic, and occupational exposures to PAHs pose an increase of risk of skin and lung cancer.2 There is compelling experimental evidence to show that benzo[a]pyrene (B[a]P) acts as a carcinogen in laboratory animals and strong epidemiologic evidence exists for a similar outcome in humans. 3 B[a]P has been studied extensively as a model for PAH carcinogenesis and was recently upgraded as a human carcinogen (group 1) by the International Agency for Research on Cancer (IARC). 3
B[a]P is chemically and biologically inert and must be metabolically activated to ultimate carcinogens which are capable of reacting with cellular macromolecules.4 Three major pathways of B[a]P metabolism has been proposed (Figure 1). The first metabolic pathway involves a oneelectron oxidation of B[a]P catalyzed by either P450 peroxidases or the monooxygenase catalytic cycle or by other peroxidases to produce a B[a]P radical cation at C6.5 The highly reactive intermediate results in the formation of several depurinating DNA adducts, mainly on C8 of Gua and N7 on Gua and Ade.6 The cation intermediate can also undergo monooxygenation to form 6- hydroxy-B[a]P which autooxidizes to yield B[a]P-3,6-dione and B[a]P-1,6-dione as signature metabolites of this pathway.
Figure 1.
Proposed B[a]P metabolic pathways and their representative metabolites. The radical cation pathway involves the activity of either P450 peroxidase or P450 monooxygenase; the diol epoxide pathway involves P450 and epoxide hydrolase; and the o-quinone pathway involves P450, epoxide hydrolase and AKR.
The second metabolic pathway involves monooxygenation of B[a]P by cytochrome P450s to yield a series of epoxides.7 The epoxide functional group can either rearrange to yield a phenol, or upon hydrolysis it is converted into the corresponding dihydrodiol intermediate. 8 The non-Kregion (−)-B[a]P-7,8-dihydrodiol can be further monoxygenated to yield (+)-anti-7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene, (+)-anti-B[a]PDE 9 which is the most mutagenic and tumorigenic diol-epoxide isomer.10 (+)-Anti-B[a]PDE is able to react with nucleophilic groups of cellular macromolecules such as proteins and DNA and induces mutagenic and cytotoxic effects in target tissues. The signature metabolite from this pathway is r-7,t-8, 9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene(B[a]P-tetrol-1). This pathway is the most well studied, and overwhelming evidence supports its importance in B[a]P induced carcinogenesis.3 Other related metabolites generated by P450s include B[a]P-7,8-dihydrodiol (B[a]P-7,8-diol), B[a]P-9,10-dihydrodiol (B[a]P-9,10-diol) and 3-OH-B[a]P. Among them, 3-OH-B[a]P was previously chosen as biomarker for biomonitoring B[a]P exposures due to its predominant formation.11
The third metabolic pathway involves the oxidation of (−)-B[a]P-7,8-dihydrodiol to yield a catechol catalyzed by dihydrodiol dehydrogenase members of the human aldo-keto reductase (AKR) superfamily (AKR1A1, AKR1C1-1C4). 12–14 The catechol is then auto-oxidized to yield B[a]P-7,8-dione which is both a Michael acceptor and redox-active.15 If the catechol and quinone are not intercepted, the quinone can enter into futile cycles generating reactive oxygen species (ROS) and oxidative stress by the depletion of NAD(P)(H). Although the formation of stable dG and dA adducts of B[a]P-7,8-dione has been reported in vitro 16–18 and the formation of the N7 depurinating Gua adduct of B[a]P-7,8-dione has been demonstrated in vitro19, this pathway is known to cause oxidative DNA damage through the transient production of ROS in human lung cells.20 The signature metabolite from this pathway is B[a]P-7,8-dione.
Since B[a]P requires metabolic activation to express its carcinogenicity via different metabolic pathways, quantitative assessment of t B[a]P metabolic profiles in exposed sites needs to be addressed. Some important questions remain unanswered. For example, how is B[a]P metabolized in susceptible tissues or species? What enzymes are involved in bioactivation vs. detoxification? And, can we regulate the activities these enzymes to prevent cancer? Our goal was therefore to develop a sensitive and robust method to quantitate B[a]P metabolic profiles in different biological settings to gain a mechanistic insight and assess the contribution of each of these metabolic pathways of B[a]P to human cancer initiation. Our methods may also provide a valid tool to monitor human PAH exposure.
Traditional analytical strategies to investigate B[a]P metabolism started with HPLC coupled with fluorescence detection.8, 21 However, this method is only applicable to selective metabolites and metabolite identity is not reliable. Hence, HPLC coupled with radiometric detection 22 was developed since it offers more precise quantitation. The limitation of this method is the availability of radiolabeled compounds and requirement of additional analytical tools to identify metabolite structures. Gas chromatography coupled to mass spectrometry (GC-MS) has been applied to study B[a]P metabolism and significantly increases the limit of detection (LOD).
However, this method only allows the detection of selected metabolites and requires timeconsuming sample pretreatment and derivatization. Alternatively, liquid chromatography coupled to mass spectrometry (LC-MS) avoids the needs for sample derivatization. In the case of metabolite quantitation, ions from the biological matrix can often confound the detection of analytes of interest. The APCI technique is less susceptible to matrix effects and ion suppression than the ESI method. Due to its high sensitivity and specificity of selected reaction monitoring (SRM) coupled with APCI-LC-MS/MS 23, we can measure precursor-product ion transitions that are specific to the analyte of interest. However, insufficient research has been conducted to quantitatively determine the B[a]P metabolome using this approach due to the limited availability of isotopically labeled B[a]P metabolite internal standards.
We have previously used HPLC coupled with radiometric detection to detect and quantitate B[a]P metabolites in human brochoalveolar H358 cells, and we have used LC-atmospheric chemical ionization (APCI)/MS/MS to obtain the identities of B[a]P metabolites. 24 With newly synthesized [13C4]-labeled B[a]P metabolites standards in hand, we have now expanded the approach to study B[a]P metabolic profiles by developing and validating a stable isotope dilution LC-APCI/SRM/MS method. Using this method, we examined the contributions of the P450 inducer-TCDD to the overall metabolism of B[a]P in the human H358 lung cell line.
Material and methods
Caution: The work described involves handling of hazardous agents and was therefore conducted in accordance with the NIH Guidelines for the Laboratory Use of Chemical Carcinogens
Reagents and Chemicals
The unlabelled B[a]P metabolite standards were purchased from the NCI Chemical Carcinogen Standard Reference Repository (Midwest Research Institute, Kansas City, MO). The [13C4]-labeled B[a]P internal standards were provided by Dr. Ronald Harvey except B[a]P-tetrol-1 (details of their synthesis are being prepared for submission elsewhere). The identities and purity of all the standards were established by LC-MS and HPLC. All solvents were HPLC grade, and all other chemicals were of the highest purity available and were used without further purification. Cell culture media were obtained from Invitrogen Co. (Carlsbad, CA) except fetal bovine serum (FBS) which was from Hyclone Laboratories (Logan, UT).
Analysis of B[a]P Metabolite Standards
A Waters Alliance 2695 chromatographic HPLC system coupled to a Waters 996 photodiode array (PDA) detector was used for the analysis of B[a]P metabolite standards. A mixture of B[a]P metabolite standards (B[a]P-tetrol-1, B[a]P-9,10-diol, B[a]P-7,8-diol, 3-OH-B[a]P, B[a]P-7,8-dione, B[a]P-1,6-dione and B[a]P-3,6-dione) were analyzed by an Agilent Zorbax ODS-C18 column, 5 µm, 4.6 mm × 250 mm (Agilent technology, Santa Clara, CA). A HPLC gradient system with solvents A (5 mM ammonium acetate in water containing 0.02% (v/v) formic acid) and B (5 mM ammonium acetate in methanol containing 0.02% (v/v) formic acid) at a flow rate of 0.5 mL/min was developed. The linear gradient increased from 55% B to 85% B over 15 min, the mobile phase was then increased to 99% B over 20 min and was held at 99% B for 20 min, the gradient was then adjusted to 55% B over 5 min.
Liquid Chromatography-Tandem Mass Spectrometric Analysis
A Finnigan TSQ Quantum Ultra spectrometer (Thermo Fisher, San Jose, CA) interfaced to a Waters Alliance 2690 HPLC system (Waters Corporation, Milford, MA) was used for the LC-MS/MS analyses. Chromatographic separation for the LC-MS/MS analyses was achieved with a Zorbax ODS-C18 column attached to a C18 guard column (Agilent Technology, Santa Clara, CA). The eluant from the HPLC column was directly introduced into a standard APCI source equipped with a corona discharge pin. Nitrogen was used as the nebulizing gas as well as the sheath gas and auxiliary gas. The vaporizer and capillary temperature were set at 500 °C and 360 °C, respectively. SRM transitions of the protonated molecules from the B[a]P metabolites were: m/z 269 to m/z 239 for B[a]P-9,10-diol, B[a]P-7,8-diol and 3-OH-B[a]P; m/z 283 to m/z 226 for B[a]P-1,6-dione, B[a]P-3,6-dione and B[a]P-7,8-dione; and m/z 285 to m/z 239 for B[a]P-tetrol-1, these m/z values were 4 amu higher for the analysis of isotopically labeled B[a]P metabolite standards, except for B[a]P-1,6-dione and B[a]P-3,6-dione where the m/z value for the SRM transition was increased by 3 amu. These reactions were monitored for selective detection of representative B[a]P metabolites from the three metabolic pathways.
Calibration Curves
To generate the calibration solutions, 10 pmol of [13C]-internal standards (B[a]P-1,6-dione, B[a]P-3,6-dione, B[a]P-7,8-dione, 3-OH-B[a]P, B[a]P-7,8-diol and B[a]P-tetrol-1) were spiked with different amounts of B[a]P-dione (0.1, 0.5, 1, 5, 10, 20 and 50 pmol), 3-OH-B[a]P (0.5, 1, 5, 10, 50, 100 and 200 pmol), B[a]P-9,10-diol (0.05, 0.1, 0.5, 1, 2, 5 and 10 pmol), B[a]P-7,8-diol (0.5, 1, 5, 10, 20, 50 and 100 pmol), and B[a]P-tetrol-1 (0.1, 0.5, 1, 5, 10, 20 and 50 pmol). Quantitative analysis of B[a]P metabolite calibration solutions was performed by a Finnigan TSQ Quantum Ultra spectrometer interfaced to a Waters Alliance 2690 HPLC system using an Aglient Zorbax ODS C18 column. The same HPLC method was used and the flow rate was set at 0.5 mL/min. The peak area ratios of the analyte : [13C]-internal standard were plotted against the amount of analytes. The linearity of the method was satisfactory in the studied range of concentrations (R2 > 0.99 for all B[a]P standards).
Cell Culture
Transformed human bronchoalveolar H358 cells (type II, epithelial origin) were obtained from American Type Culture Collection (ATCC #CRL-5807) and maintained in RPMI 1640 culture medium with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 units/mL penicillin and 100 µg/mL streptomycin. All cells were maintained in 10 cm dishes and were incubated at 37 °C in a humidified incubator maintained at 5% CO2 and culture media was renewed every 2–3 days. The cells were sub-cultured every 6–7 days using 0.25% trypsin. Cultured cells with a passage number of 10–20 were used in the experiments to reduce variability.
B[a]P metabolism in H358 Cells
The metabolism of B[a]P in H358 cells was conducted according to published procedures. 24 Briefly, the cells (~ 2 × 107) were induced with TCDD (10 nM) in 10 mL of RPMI/FBS medium for 15 h before B[a]P treatment (4 µM, 0.5% DMSO) in 3 mL of HBSS (10 mM glucose). Control experiments were performed in an identical manner with the exception that DMSO was substituted for TCDD. Before the treated cells were harvested, a [13C4]-B[a]P metabolite standard cocktail (containing 100 pmoles each of analyte except [13C4]-B[a]P-7,8-diol was 50 pmol) was added to cell culture dishes to normalize for losses in the sample workup and analysis. Cells were sampled at 0, 3, 6, 12 and 24 h. The total cell culture mixture was harvested by a cell scraper into a 10 mL tube and extracted twice with 3 mL of water-saturated ethyl acetate. The combined organic extract was dried under vacuum and the residue was redissolved in 100 µL of H2O/MeOH (1:1) and put into an autosampler vial for LC-MS analysis. All incubations were performed in triplicate in two independent experiments.
Results
Development of Liquid Chromatographic Conditions
Previously we reported a reversed phase HPLC method which separated all the major B[a]P metabolites over 90 min.24 This method was coupled to an in-line UV and radiometric detector to identify and quantitate these metabolites. To take full advantage of the superior sensitivity of the mass spectrometer detector, a shorter chromatographic method with good separation power was required. We injected B[a]P metabolite standards individually as well as a cocktail of mixed standards into a Zorbax ODS C18 column to optimize shorter-run conditions. Satisfactory chromatographic separation of B[a]P metabolites was obtained using 5mM ammonium acetate in water plus 0.02% (v/v) formic acid as mobile phase A and 5mM ammonium acetate in methanol plus 0.02% (v/v) formic acid as mobile phase B. This mobile phase is compatible with LC-MS analysis due to the volatility of ammonium acetate. All B[a]P standards (20 pmol) of interest were well separated in a 60 min gradient as illustrated in Figure 2.
Figure 2.
HPLC-UV chromatogram of a mixture of B[a]P metabolite standards monitored at 254 nm.
Development of Mass-Spectral Detection
Both electrospray ionization and APCI modes have been tested for detecting B[a]P metabolites; it was found that the APCI was more sensitive and therefore adopted in our study.25 Direct infusions of metabolite standards (B[a]P-tetrol-1, B[a]P-7,8-diol, B[a]P-9,10-diol, B[a]P-1,6-dione, B[a]P-3,6-dione, B[a]P-7,8-dione, B[a]P-3-OH) were performed to optimize ionization parameters for their identification. Full scan spectra of all the hydroxylated (mono-, di- or tetra-OH) standards under positive APCI conditions were dominated by dehydrated precursors, which likely resulted from a gas-phase dehydration process involving loss of H2O. Since these precursor ions were stable they were chosen as parent ions in SRM analysis of those metabolites. The parent ion for B[a]P-tetrol-1 was m/z 285 [M+H−2H2O]+, and the product ion was m/z 239 resulting from a further loss of H2O and CO, the collision energy was set at 25 v. For B[a]P-7,8-diol and B[a]P-9,10-diol the parent ion was m/z 269 [M+H−H2O]+ and the product ion was m/z 239 resulting from a further loss of CH2O26; and for 3-OH-B[a]P the parent ion was m/z =269 and the product ion was m/z 239 resulting from a loss of CH2O; and in each case the collision energy was set at 44 v. For B[a]P-diones, the most abundant parent ion was m/z 283 [M+H]+ and the most abundant product ion was m/z 226 (loss of two CO), and the collision energy was set at 40 v. Coupled with the modified HPLC method, a mixture of 20 pmol of each B[a]P metabolite standard was injected onto the column connected to an APCI-MS ion source. Metabolites were detected by SRM using a TSQ mass spectrometer. Representative LC-SRM/MS chromatograms which demonstrate excellent separation and detection for all the analytes are shown in Figure 3.
Figure 3.
Representative LC-MS chromatograms of a mixture of B[a]P metabolite standards. *low collision energy was used with the loss of one CO was monitored
Development of Stable-Isotope Dilution Method
The key components to develop a stable isotope dilution LC-SRM/MS method was to secure isotopically labeled B[a]P internal standards. We developed two different synthetic strategies to incorporate four 13C atoms to the B[a]P structure as shown in Figure 4, which involved sequential palladium-catalyzed cross-coupling reactions (manuscripts in preparation). Each synthetic strategy incorporated the four atoms of 13C into different positions in the B[a]P-ring, see compounds listed in the left and right columns of Figure 4. Individual B[a]P metabolites of interest thus contained [13C4] incorporated into one of the two sets of the positions indicated. Based on the SRM information of unlabeled B[a]P standards and similar direct infusions for [13C4]-labeled B[a]P standards, the transitions for [13C4]-B[a]P metabolites were chosen: m/z 289 to m/z 243 for [13C4]-B[a]P-tetrol-1; m/z 273 to m/z 243 for [13C4]-B[a]P-7,8-diol and [13C4]-3-OH-B[a]P; and m/z 287 to m/z 230 for [13C4]-B[a]P-7,8-dione. Changing collision energy significantly influences the choice of mass transition for the dione series. Elevated collision energy produces increased amounts of product ions derived from the loss of two CO, which displayed higher sensitivity compared to lower collision energy in which loss of only one CO was dominant. Since one of the 13C atoms is at the C6 position, the loss of two CO needs to take into consideration the loss of the labeling from the internal standards, so for [13C4]-B[a]P-1,6-dione and [13C4]-B[a]P-3,6-dione, the mass transition monitored was chosen from m/z 287 to m/z 229 [12C=O; 13C=O]. Therefore, six B[a]P metabolite internal standards were premixed and injected into LC-APCI/MS. Good separation and sensitive detection of all the labeled internal standards was obtained and the corresponding channels for unlabelled compound were devoid of any cross-over.
Figure 4.
Two sets of [13C4]-labeled B[a]P metabolite standards showing the position of 13C-incorporation.
A set of seven standard curve solutions were prepared by spiking different amounts of B[a]P metabolite standard with a constant amount of synthesized [13C4]-labeled internal standard and the amounts were analyzed by LC-MS/MS. The peak area ratios of analyte and corresponding isotope labeled internal standard were plotted against the added amount of analyte (Figure 5). B[a]P-9,10-diol is a characteristic P450 metabolite, and we do not have its isotope labeled internal standard, so we used the structurally similar [13C4]-B[a]P-7,8-diol as its internal reference to construct the seventh standard curve. For all standards analyzed, excellent linearity was found with R2 ≥0.99. The absolute amount of analyte at the lower end of the linear range, calculated from the lowest standard concentration included in the calibration curve with the signal noise ratio >3, was defined as the lower limit of detection (LOD). The limit of quantitation (LOQ) was determined with the signal noise ratio > 10. The LOD for compounds B[a]P-7,8-diol and 3-OH-B[a]P was 1.5 fmol injected onto the column and LOQ was 6 fmol on column for both compounds. The LOD and LOQ for all the other metabolites were all in the lower fmol range (Table 2). The accuracy of the method was tested by spiking known amounts of each metabolite into cell culture media. The precision of the method for each metabolite was determined by calculating the coefficient of variation of five measurements of the standards. Accuracy and precision estimates for all metabolites are summarized in Table 2. The accuracies vary from 96% to 105% and coefficients of variation of are under 8% for most analytes.
Figure 5.
Representative standard curves performed in MeOH and in the cell matrices. The solid triangle represents the regression line for samples prepared in MeOH, the cross represents the regression lines for samples prepared in matrices and the solid square represents the expected regression lines for samples with 100% recovery. (A) B[a]P-tetrol-1/[13C4]-B[a]P-tetrol-1, (r2 = 0.9972 for MeOH and r2 = 0.9965 for matrices); (B) 3-OH-B[a]P/[13C4]-3-OH-B[a]P (r2 = 0.999 for MeOH and r2 = 0.9986 for matrices) and (C) B[a]P-7,8-dione/[13C4]-B[a]P-7,8-dione (r2 =0.9977 for MeOH and r2 = 0.9946 for matrices).
Table 2.
Accuracy, precision and LOD/LOQ of the LC-MS method.
| Analyte | Accuracy (%) | Precision (%) | LOD/LOQ (fmol) |
|---|---|---|---|
| B[a]P-tetrol-1 | 99.9 ± 7.5 | 5.83 | 6.0/10.0 |
| B[a]P-7,8-diol | 101.2 ± 5.2 | 5.22 | 1.5/6.0 |
| 3-OH-B[a]P | 104.8 ± 6.6 | 6.26 | 1.5/6.0 |
| B[a]P-7,8-dione | 96.2 ± 9.1 | 11.64 | 3.0/6.0 |
| B[a]P-1,6-dione | 98.8 ± 4.4 | 2.55 | 3.0/6.0 |
| B[a]P-3,6-dione | 102.1 ± 6.6 | 2.42 | 3.0/6.0 |
To estimate the matrix effect, stock solutions of B[a]P standards along with [13C4]-labeled internal standards were added to the cell incubation media and worked up as stated previously. The calibration curves in media for each standard were superimposable with those obtained from H2O/MeOH (Figure 5). However, the recovery of each metabolite differed. For 3-OH-B[a]P, the recovery was close to 86% in media vs. H2O/MeOH, while with B[a]P-7,8-dione, the recovery was only 10% (Table 1). The significant loss of B[a]P-7,8-dione may be due to its reactivity with nucleophiles after addition to cell incubation media.
Table 1.
Average recovery of stable isotope-labeled B[a]P metabolites used as internal standards
| Internal standard | Recovery (%) |
|---|---|
| B[a]P-tetrol-1 | 76.0 |
| B[a]P-7,8-diol | 67.9 |
| 3-OH-B[a]P | 86.1 |
| B[a]P-7,8-dione | 8.5 |
| B[a]P-1,6-dione | 53.9 |
| B[a]P-3,6-dione | 72.7 |
Application of Stable-Isotope Dilution LC-MS Methodology to Measure B[a] P metabolome in Human Lung Cells
H358 cells (~ 2 × 107) in 3 mL of media were incubated with 4 µM of B[a]P in 10 cm dishes. Cells were either pretreated with TCDD (10 nM) to induce cytochrome P450 or with DMSO prior to the addition of 12 nmol of B[a]P (finial concentration 4 µM). After the addition of B[a]P, the total cell culture mixture was removed at 0, 3, 6, 12, and 24 h, a cocktail of [13C4]-B[a]P metabolite standards was added and the sample and extracted with ethyl acetate for MS analysis.
The total organic metabolites formed over 24 h never exceeded 3 nmol (Figure 6), compared with 12 nmol dose of B[a]P given to the cells. Importantly, pretreatment of the cells with TCDD led to a more rapid appearance of organic soluble metabolites. It was found that a significant amount of parent compound disappeared during the first 6 h and at the end of 24 h, only a small amount of B[a]P remained (data not shown). During this time, B[a]P was converted to water soluble conjugates, such as glucuronides or sulfates, and could not be extracted into the organic phase. To verify this, aqueous extracts from H358 cell incubations were hydrolyzed by β-glucuronidase type H-1, which also has sulfatase activity. 3-OH-B[a]P was detected from the media extract indicating the formation of water soluble conjugates of 3-OH-B[a]P (data not shown). The amount of 3-OH-B[a]P released is significant and could be used to account for a large part of B[a]P metabolites in the aqueous phase. These data were consistent with our earlier findings in these cells.24
Figure 6.
Distribution of total B[a]P organic metabolites in H358 cells over 24h after pretreatment with TCDD (A) or without TCDD (B). The mean ± SD for n=3 is shown.
The formation of signature metabolites characteristic of each metabolic pathway was measured by our stable isotope dilution mass spectrometry method. A representative LC-SRM/MS chromatogram of DMSO treated H358 cells incubated with B[a]P at 12 h is illustrated in Figure 7. The formation of individual metabolites was clearly seen in one of the three channels. The time course for the appearance of each metabolite were determined (Figure 8). Signature metabolites from the radical cation pathway (B[a]P-1,6-dione, B[a]P-3,6-dione), the diolepoxide pathway (B[a]P-tetrol-1) and the o-quinone pathway (B[a]P-7,8-dione) were all detected in the organic extracts. Other P450 mediated metabolites (3-OH-B[a]P, B[a]P-7,8-diol and B[a]P-9,10-diol) were also detected. The most abundant metabolite was 3-OH-B[a]P, which has been used as a biomarker for B[a]P exposure. The time courses of formation of metabolites using the newly developed stable isotope dilution mass spectrometry method are in reasonable agreement with our previous study using [3H]-B[a]P.24 The major difference was in the amount of B[a]P-7,8-dione, which was now quantitated in significantly higher amount than previously reported by the radiochemical method. It is important to note that the B[a]P-tetrol-1 and B[a]P-7,8-dione were produced in comparable amounts with or without TCDD induction. As the level of the signature metabolites formed from the radical cation, diol-epoxide and o-quinone pathway are produced in similar amounts, it implies that they play similar roles in B[a]P metabolism in H358 cells.
Figure 7.
Representative 12 h time point of LC/MS/MS chromatograms of B[a]P metabolites from extraction of TCDD treated H358 cells incubated with 4 µM B[a]P. Injection volume was 10 µL. A) B[a]P tetrol-1 (14.6 min); B) B[a]P-9,10-diol (17.2 min), B[a]P-7,8-diol (24.5 min) and 3-OH-B[a]P (35.6 min); C) B[a]P-7,8-dione (27.9 min), B[a]P-1,6-dione (32.2 min) and B[a]P-3,6-dione (33.1 min). CM: cellular metabolite; IS: [13C4]-labeled internal standard.
Figure 8.
Appearance of B[a]P major metabolites over 24 h (± TCDD). A). B[a]P-tetrol-1; B). B[a]P-9,10-diol; C). B[a]P-7,8-diol; D). 3-OH-B[a]P; E). B[a]P-7,8-dione; F). B[a]P-1,6-dione; G). B[a]P-3,6-dione. Solid triangle represents metabolites from TCDD treated cells and solid circle represents metabolites from DMSO treated cells. The mean ± SD is shown n=3.
Discussion
Using a unique library of [13C4]-labeled B[a]P internal standards, we developed a stable isotope dilution tandem mass spectrometry method which is superior for the analysis of B[a]P metabolic profiles. The addition of a known quantity of labeled standards at the earliest possible stage compensates for variations in sample preparation, injection or matrix effects. Characteristic SRM transitions were chosen to monitor representative B[a]P metabolites. This method displays high sensitivity, and LOD of 1.5 fmol was achieved for B[a]P-7,8-diol and 3-OH-B[a]P. It also enables a simultaneous and accurate quantification of all major B[a]P metabolites. The improved sensitivity provides a method to assess the contributions of different pathways to the metabolic activation of B[a]P in a variety of settings.
Earlier approaches to measure B[a]P metabolism were based on chromatographic separation followed by fluorescence detection. Krahn et al. adopted this method to quantitate PAH metabolites by measuring fluorescence at 380/430 nm excitation/emission,27 and a LOD of 400 fmol was reported for 1-OH-pyrene. This method provided important information about B[a]P metabolism, however, the entire B[a]P metabolome could not be measured in one experiment, since B[a]P-diones are non-fluorescent. Additionally, in line fluorescence spectral acquisition does not provide structural identity of analyte. This method was replaced by using radioistopically labeled B[a]P to measure metabolites by liquid chromatography coupled with inline radioactive detection. Jiang et al. studied the metabolism of [3H]-B[a]P in H358 cells and a comprehensive analysis of B[a]P metabolites was accomplished using this method. 24 This method had a LOD estimated to be 1 pmol. However, the major limitation of those methods is that an independent analytical method had to be used for the unequivocal identification of the metabolites.
The MS detector has seen increasing application in studies of B[a]P metabolism. GC-MS was introduced to monitor volatile PAHs or their volatile derivatives. Unfortunately this method involves multiple derivatization and purification steps that render it extremely laborious. LC/MS offers an alternative to examine polar compounds directly without the limitations occurred in GC-MS. The ion source of choice for the detection of B[a]P and its derivatives is APCI since it provides higher sensitivity compared with ESI. Combined with tandem mass spectrometry method, it provides an accurate and facile method to identify B[a]P metabolites. Wang et al. monitored metabolites in urine of mice exposed to B[a]P, using a LC-QTOF-MS method, their LOD ranges from 150 to 200 fmol.28 Fan et al. reported a LC-MS/MS method to detect only 3-OH-B[a]P with a LOD of 50 fmol.26 In another report, A LOD of 20 fmol was reported for B[a]P-7,8-diol based on a LC-APCI method.25 Our method is superior to these earlier reports.
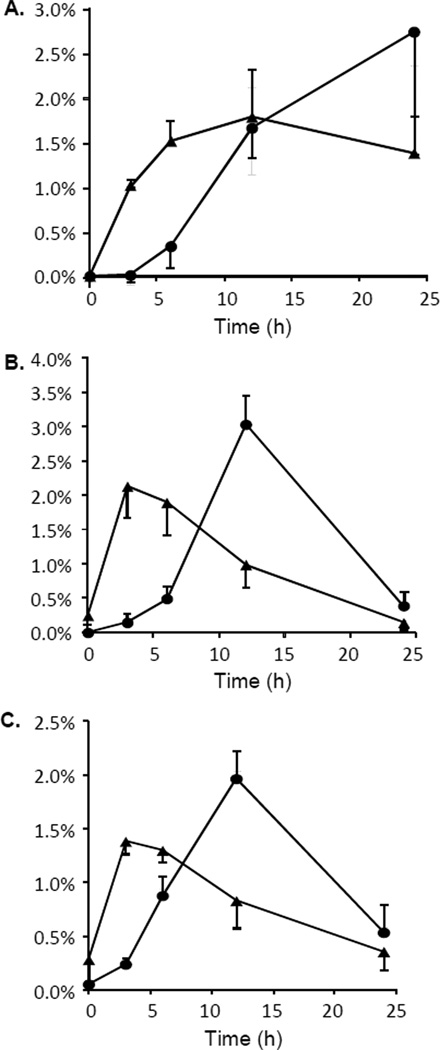
Our new method was used to measure B[a]P metabolic profiles in H358 cells, in which one group was treated with TCDD, an inducer for cytochrome P450 enzymes, and the control group was treated with DMSO. Naïve H358 cells do not constitutively express cytochrome P450 1A1/1B1 or AKRs, which are thought to play an important role in B[a]P metabolism.29 TCDD is a potent ligand of the aryl hydrocarbon receptor (AhR) and the activated receptor is capable of inducing CYP1B1 in H358 cells. We previously showed that incubation of H358 cells with B[a]P also led to the induction of AKR1C1 and proposed that the earlier appearance of B[a]P- 7,8-dione following TCDD pre-treatment likely resulted from the formation or the requisite electrophilic B[a]P metabolite to induce the anti-oxidant response element on the AKR1C1 promoter. 24 The highest level of B[a]P metabolites identified were hydroxylated B[a]P derivatives, such as 3-OH-B[a]P, B[a]P-7,8-diol and B[a]P-9,10-diol, which involve P450 mediated metabolism. By 24 h the difference between the two treatment groups was less prominent and agrees with the known concept that B[a]P induces its only metabolism by binding to AhR.30 The time course profiles in this study are in general agreement with our previous study with the exception of B[a]P-7,8-dione. 24 We reasoned that the radiometric detector only detected readily extracted B[a]P-7,8-dione and may not account for the amount produced during the metabolism of B[a]P because of its reactivity. In our newly developed stable isotope dilution mass spectrometry method, the loss of B[a]P-7,8-dione was normalized by [13C4]-labeled B[a]P-7,8-dione. Therefore, the real amount of B[a]P-7,8-dione was better measured by the current method which was now found to be present in greater amounts. Importantly, comparable amounts of signature metabolites generated from the radical cation pathway (B[a]P-1,6-dione and B[a]P-3,6-dione ), diol epoxide pathway (B[a]P-tetrol-1) and o-quinone pathway (B[a]P-7,8-dione) in the TCDD and DMSO treated group indicates similar contributions of all three pathways to PAH metabolism in the H358 cell line (Figure 9). The current method provides a quantitative tool to fingerprint the three metabolic pathways of B[a]P in any biological setting and help determine their relationship to B[a]P metabolism and human cancer risk.
Figure 9.
Contribution of the three metabolic pathways of B[a]P metabolism in H358 cells. A). radical cation pathway, (B[a]P-1,6-dione and B[a]P-3,6-dione); B). diol-epoxide pathway, (B[a]P-tetrol-1); C). o-quinone pathway, (B[a]P-7,8-dione). Solid triangle represents metabolites from TCDD treated cells and solid circle represents metabolites from DMSO treated cells. The mean ± SD is shown n=3.
Assessment of human health risk to PAH exposure is an important issue and a key component is to identify effective biomarkers and efficient analytical tools. The complex nature of PAH mixtures and their variability poses unique challenges. Urinary PAH metabolites can be conveniently detected by multiple analytical techniques, and many of them have received considerable attention to characterize PAH exposure 31. For example, pyrene is present in most PAH mixtures and levels of 1-hydroxypyrene excreted in human urine were found to increase linearly with airborne concentrations of pyrene.32 Hence, 1-hydroxypyrene has been used extensively as a biological indicator of PAH exposure in human populations. However, pyrene itself is not carcinogenic and no bay-region diol epoxide is formed during its biotransformation
Phenanthrene (Phe) is the simplest PAH to contain a bay region, and the formation of the diol epoxide is one pathway of metabolism of this compound. Predominant excretion of Phe is found in urine and its metabolites include phenols, dihydrodiols, and tetrols. It is noted that Phe is not a human carcinogen, but its metabolism shows close similarity to that of the more carcinogenic B[a]P and similar enzymes are likely to be involved in the biotransformation of both PAHs 33. Phe tetrols have been proposed to be biomarkers of PAH exposure and several studies have demonstrated their utility. 34, 35 Without enantiomeric separation, Phe tetrols were shown to be good indicators for PAH exposure, especially if the diol epoxide metabolic pathway dominates the resulting carcinogenicity and toxicity of the PAH.36
The validity of using urinary metabolites of pyrene or Phe as surrogate biomarker for real exposure assessment of carcinogenic PAHs has been criticized since these compounds are not carcinogenic. Consequently, attempts have been made to validate effective biomarkers from other relevant and toxic PAHs. B[a]P has been demonstrated to be one of the most potent carcinogens among PAHs and is virtually detected in all PAH mixtures. Therefore, its metabolites have been investigated as biomarkers of PAH exposure. 3-OH-B[a]P is one of the major metabolites selected to monitor PAH carcinogenic risk and several studies have successfully demonstrated its value. 37, 38 The limitation is that only low concentrations of 3-OH-B[a]P are excreted in urine (1000–10,000 times lower than 1-OHP) and sensitive analytical tools are needed for its detection. Other choices of biomarker of B[a]P include the tetrols that came from diol epoxide pathway. 39, 40 We reasoned that a metabolic profile could provide a more comprehensive understanding on B[a]P exposure and effect since it could contain signature metabolites of each of the metabolic pathways of activation: B[a]P-1,6- dione, B[a]P-3,6-dione (radical cation) B[a]P-tetrol-1 (diol-epoxide), and B[a]P-7,8-dione (o-quinone), Our newly developed method was able to detect signature B[a]P metabolites simultaneously with improved sensitivity which will increase our ability to perform effective biomonitoring of PAHs in human urine and plasma.
Conclusions
We describe the development and application of an analytical method to measure B[a]P metabolic profiles. An efficient HPLC method was developed which separated the major metabolites. An LC-APCI/SRM/MS method was then developed to selectively detect and quantitate those metabolites. Using this method, the most abundant metabolites, 3-OH-B[a]P and B[a]P-7,8-diol could be detected with a LOD of 1.5 fmol on column. We applied this method to measure the B[a]P metabolome in human H358 cells and showed that the contributions of the radical cation, diol-epoxide and o-quinone pathways to B[a]P activation were equivalent Because different cells metabolize B[a]P differently, quantitation of major metabolites derived from different metabolic pathways will not only help understand contributions of different pathways to overall B[a]P activation and deactivation, but also reveal the biomarkers for B[a]P exposure at the cellular level. It will also improve the power to assess human B[a]P exposure and effect and improve a cancer risk assessment.
Acknowledgments
Funding Support
This work was supported in part by RO1ES015857, P30ES013508 and PADOH 4100038714 awarded to TMP, RO1CA130038 awarded to IAB.
Abbreviations
- AhR
aryl hydrocarbon receptor
- AKR
aldo-keto reductase
- APCI
atmospheric chemical ionization
- (+)-anti-B[a]PDE
7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene
- LOD
limit of detection
- LOQ
limit of quantitation
- PAH
polycyclic aromatic hydrocarbon
- SRM
selected reaction monitoring
- TCDD
2,3,7,8 -tetrachlorodibenzo-p-dioxin
References
- 1.IARC-Monographs. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 32: Polynuclear Aromatic Compounds, Pt. 1: Chemical, Environmental and Experimental Data. 1983 [PubMed] [Google Scholar]
- 2.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis (London) 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 3.IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 4.Conney AH, Chang RL, Jerina DM, Wei SJC. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 1994;26:125–163. doi: 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri EL, Rogan EG. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica. 1995;25:677–688. doi: 10.3109/00498259509061885. [DOI] [PubMed] [Google Scholar]
- 6.Stack DE, Cremonesi P, Hanson A, Rogan EG, Cavalieri EL. Radical cations of benzo[a]pyrene and 6-substituted derivatives: reaction with nucleophiles and DNA. Xenobiotica. 1995;25:755–760. doi: 10.3109/00498259509061890. [DOI] [PubMed] [Google Scholar]
- 7.Bauer E, Guo Z, Ueng YF, Bell LC, Zeldin D, Guengerich FP. Oxidation of benzo[a]pyrene by recombinant human cytochrome P450 enzymes. Chem. Res. Toxicol. 1995;8:136–142. doi: 10.1021/tx00043a018. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR. Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis (London) 1998;19:1847–1853. doi: 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- 9.Cooper CS, Grover PL, Sims P. The metabolism and activation of benzo[a]pyrene. Prog. Drug Metab. 1983;7:295–396. [Google Scholar]
- 10.Kapitulnik J, Wislocki PG, Levin W, Yagi H, Jerina DM, Conney AH. Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (+)-trans-7b, 8a-dihydroxy-9a,10a-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res. 1978;38:354–358. [PubMed] [Google Scholar]
- 11.Simon P, Lafontaine M, Delsaut P, Morele Y, Nicot T. Trace determination of urinary 3-hydroxybenzo[a]pyrene by automated column-switching high-performance liquid chromatography. J. Chromatogr., B: Biomed. Sci. Appl. 2000;748:337–348. doi: 10.1016/s0378-4347(00)00350-9. [DOI] [PubMed] [Google Scholar]
- 12.Burczynski ME, Harvey RG, Penning TM. Expression and Characterization of Four Recombinant Human Dihydrodiol Dehydrogenase Isoforms: Oxidation of trans-7,8-Dihydroxy-7,8-dihydrobenzo[a]pyrene to the Activated o-Quinone Metabolite Benzo[a]pyrene-7,8-dione. Biochemistry. 1998;37:6781–6790. doi: 10.1021/bi972725u. [DOI] [PubMed] [Google Scholar]
- 13.Palackal NT, Burczynski ME, Harvey RG, Penning TM. The ubiquitous aldehyde reductase (AKR1A1) oxidizes proximate carcinogen transdihydrodiols to o-quinones: potential role in polycyclic aromatic hydrocarbon activation. Biochemistry. 2001;40:10901–10910. doi: 10.1021/bi010872t. [DOI] [PubMed] [Google Scholar]
- 14.Palackal NT, Lee SH, Harvey RG, Blair IA, Penning TM. Activation of polycyclic aromatic hydrocarbon trans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional over-expression in human lung carcinoma (A549) cells. J. Biol. Chem. 2002;277:24799–24808. doi: 10.1074/jbc.M112424200. [DOI] [PubMed] [Google Scholar]
- 15.Penning TM, Ohnishi ST, Ohnishi T, Harvey RG. Generation of Reactive Oxygen Species during the Enzymic Oxidation of Polycyclic Aromatic Hydrocarbon trans-Dihydrodiols Catalyzed by Dihydrodiol Dehydrogenase. Chem. Res. Toxicol. 1996;9:84–92. doi: 10.1021/tx950055s. [DOI] [PubMed] [Google Scholar]
- 16.Shou M, Harvey RG, Penning TM. Reactivity of benzo[a]pyrene-7,8-dione with DNA. Evidence for the formation of deoxyguanosine adducts. Carcinogenesis (London) 1993;14:475–482. doi: 10.1093/carcin/14.3.475. [DOI] [PubMed] [Google Scholar]
- 17.Balu N, Padgett WT, Lambert GR, Swank AE, Richard AM, Nesnow S. Identification and Characterization of Novel Stable Deoxyguanosine and Deoxyadenosine Adducts of Benzo[a]pyrene-7,8-quinone from Reactions at Physiological pH. Chem. Res. Toxicol. 2004;17:827–838. doi: 10.1021/tx034207s. [DOI] [PubMed] [Google Scholar]
- 18.Balu N, Padgett WT, Nelson GB, Lambert GR, Ross JA, Nesnow S. Benzo[a]pyrene-7,8-quinone-3'-mononucleotide adduct standards for 32P postlabeling analyses: Detection of benzo[a]pyrene-7,8-quinone-calf thymus DNA adducts. Anal. Biochem. 2006;355:213–223. doi: 10.1016/j.ab.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 19.McCoull KD, Rindgen D, Blair IA, Penning TM. Synthesis and Characterization of Polycyclic Aromatic Hydrocarbon o-Quinone Depurinating N7-Guanine Adducts. Chem. Res. Toxicol. 1999;12:237–246. doi: 10.1021/tx980182z. [DOI] [PubMed] [Google Scholar]
- 20.Park J-H, Mangal D, Tacka KA, Quinn AM, Harvey RG, Blair IA, Penning TM. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6846–6851. doi: 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barhoumi R, Catania JM, Parrish AR, Awooda I, Tiffany-Castiglioni E, Safe S, Burghardt RC. Multiphoton spectral analysis of benzo[a]pyrene uptake and metabolism in breast epithelial cell lines. J. Toxicol. Sci. 2009;34:13–25. doi: 10.2131/jts.34.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James MO, Altman AH, Morris K, Kleinow KM, Tong Z. Dietary modulation of phase 1 and phase 2 activities with benzo(A)pyrene and related compounds in the intestine but not the liver of the channel catfish, Ictalurus punctatus. Drug Metab. Dispos. 1997;25:346–354. [PubMed] [Google Scholar]
- 23.Ciccimaro E, Blair IA. Stable-isotope dilution LC-MS for quantitative biomarker analysis. Bioanalysis. 2010;2:311–341. doi: 10.4155/bio.09.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Gelhaus SL, Mangal D, Harvey RG, Blair IA, Penning TM. Metabolism of Benzo[a]pyrene in Human Bronchoalveolar H358 Cells Using Liquid Chromatography-Mass Spectrometry. Chem. Res. Toxicol. 2007;20:1331–1341. doi: 10.1021/tx700107z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koeber R, Niessner R, Bayona JM. Comparison of liquid chromatography-mass spectrometry (LC/MS) interfaces for the analysis of polar metabolites of benzo[a]pyrene. Fresenius' J. Anal. Chem. 1997;359:267–273. [Google Scholar]
- 26.Fan R, Dong Y, Zhang W, Wang Y, Yu Z, Sheng G, Fu J. Fast simultaneous determination of urinary 1-hydroxypyrene and 3-hydroxybenzo[a]pyrene by liquid chromatography-tandem mass spectrometry. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2006;836:92–97. doi: 10.1016/j.jchromb.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Krahn MM, Burrows DG, MacLeod WD, Jr, Malins DC. Determination of individual metabolites of aromatic compounds in hydrolyzed bile of English sole (Parophrys vetulus) from polluted sites in Puget Sound, Washington. Arch. Environ. Contam. Toxicol. 1987;16:511–522. doi: 10.1007/BF01055807. [DOI] [PubMed] [Google Scholar]
- 28.Wang JJ, Frazer DG, Law B, Lewis DM. Identification and quantification of urinary benzo[a]pyrene and its metabolites from asphalt fume exposed mice by microflow LC coupled to hybrid quadrupole time-of-flight mass spectrometry. Analyst (Cambridge, U. K.) 2003;128:864–870. doi: 10.1039/b302617p. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Shen Y-M, Quinn AM, Penning TM. Competing Roles of Cytochrome P450 1A1/1B1 and Aldo-Keto Reductase 1A1 in the Metabolic Activation of (+−)-7,8-Dihydroxy-7,8-dihydro-benzo[a]pyrene in Human Bronchoalveolar Cell Extracts. Chem. Res. Toxicol. 2005;18:365–374. doi: 10.1021/tx0497245. [DOI] [PubMed] [Google Scholar]
- 30.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 31.Jacob J, Seidel A. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2002;778:31–47. doi: 10.1016/s0378-4347(01)00467-4. [DOI] [PubMed] [Google Scholar]
- 32.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies - a review. Int. J. Hyg. Environ. Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Shou M, Korzekwa KR, Krausz KW, Crespi CL, Gonzalez FJ, Gelboin HV. Regio- and stereo-selective metabolism of phenanthrene by twelve cDNAexpressed human, rodent, and rabbit cytochromes P-450. Cancer Lett. (Shannon, Irel.) 1994;83:305–313. doi: 10.1016/0304-3835(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 34.Carmella SG, Chen M, Yagi H, Jerina DM, Hecht SS. Analysis of Phenanthrols in Human Urine by Gas Chromatography-Mass Spectrometry: Potential Use in Carcinogen Metabolite Phenotyping. Cancer Epidemiol., Biomarkers Prev. 2004;13:2167–2174. [PubMed] [Google Scholar]
- 35.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in Human Urine: A Potential Biomarker for Assessing Polycyclic Aromatic Hydrocarbon Metabolic Activation. Cancer Epidemiol., Biomarkers Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 36.Hochalter JB, Zhong Y, Han S, Carmella SG, Hecht SS. Quantitation of a Minor Enantiomer of Phenanthrene Tetraol in Human Urine: Correlations with Levels of Overall Phenanthrene Tetraol, Benzo[a]pyrene Tetraol, and 1-Hydroxypyrene. Chem. Res. Toxicol. 2011;24:262–268. doi: 10.1021/tx100391z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jongeneelen FJ, Anzion RBM, Henderson PT. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J. Chromatogr., Biomed. Appl. 1987;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- 38.Onyemauwa F, Rappaport SM, Sobus JR, Gajdosova D, Wu Ra, Waidyanatha S. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2009;877:1117–1125. doi: 10.1016/j.jchromb.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 39.Simpson CD, Wu M-T, Christiani DC, Santella RM, Carmella SG, Hecht SS. Determination of r-7,t-8,9,c-10-Tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in Human Urine by Gas Chromatography/Negative Ion Chemical Ionization/Mass Spectrometry. Chem. Res. Toxicol. 2000;13:271–280. doi: 10.1021/tx990202c. [DOI] [PubMed] [Google Scholar]
- 40.Wu M-T, Simpson CD, Christiani DC, Hecht SS. Relationship of exposure to coke-oven emissions and urinary metabolites of benzo(a)pyrene and pyrene in coke-oven workers. Cancer Epidemiol., Biomarkers Prev. 2002;11:311–314. [PubMed] [Google Scholar]