Abstract
Background and purpose
The human X chromosome is enriched with testis-specific genes that may be crucial for male fertility. Mutations in USP26 gene have been proposed to be associated with male infertility. Moreover, the importance of the ubiquitin pathway during different stages of mammalian fertilization and even embryo development has been addressed. Some mutations and haplotypes on this gene have been proposed to be associated with male infertility. In this study, five different mutations on USP26 were investigated: 1737 G > A, 1090 C > T, 370-371ins ACA, 494 T > C and 1423 C > T.
Methods
The study included 166 infertile men with non-obstructive azoospermia, 72 male partners of couples who had previously experienced ≥3 clinical first trimester spontaneous abortions and 60 fertile men. Besides family history of reproduction, hormonal evaluation and semen analysis were performed. DNA was extracted from blood samples. PCR-SSCP, PCR-RFLP and PCR Product Cloning methods were used and resumed by sequencing to insure about the mutations. Moreover, USP26 gene expression was studied by Real-Time PCR after RNA extraction followed by cDNA synthesis from 24 testis biopsies in obstructive and non-obstructive azoospermia patients.
Results
The results indicate that there is a haplotype between three observed mutations in Iranian population include: 370-371insACA, 1423C > T and 494 T > C. This haplotype was seen in control group as well. Surprisingly, total frequency of mutations in men with history of idiopathic RPL and azoospermic cases were significantly higher than that of in control groups (p < 0.05). Serum testosterone concentrations and testicular volume did not differ in the mutation positive group compared with the non-mutation group. About the USP26 gene expression, there is a significant difference between the expression levels of obstructive azoospermia, complete maturation arrest samples and SCO samples (P < 0.05).
Conclusions
According to our results, the USP26 gene may play an important role in male reproduction. The alterations of this gene may be involved in male infertility and RPL in Iranian population and may negatively affect testicular function.
Keywords: Polymorphism, Male infertility, USP26
Introduction
Infertility affects 10 %–15 % of couples, with 20 % of such cases being caused by pure male factor infertility [1, 2]. Although there are many factors that contribute to male infertility, in nearly 50 % of infertile men the cause cannot be identified and this situation has been defined as unexplained or idiopathic, however some causes of male infertility have been determined [2]. Some studies suggest that impaired spermatogenesis is an essential etiology of male infertility and that genetic disorders affecting spermatogenesis might be responsible for many cases of idiopathic infertility [3]. In recent years, a lot of attention has been paid to genetic causes of male infertility. Yq microdeletions have been detected in 5 %–15 % of males with spermatogenic defect using specific sequence-tagged sites (STS) in long arm of Y chromosome [4]. In addition to Y chromosome microdeletion and mutation of autosome genes, X chromosomes are also closely related to male fertility; however, the underlying molecular mechanisms are still unknown [3, 5]. There has been an intensive search in such genes located on the X chromosome as men are hemizygous for this chromosome [6, 7]. Nishimune et al. [8] observed many genes on the X chromosome that are related to male infertility. One of those genes is ubiquitin-specific protease 26 (USP26), first identified from a screen for X-linked genes involved in spermatogenesis by Wang et al. [9], who confirmed the expression of USP26 RNA in mice testis. USP26 belongs to a family of deubiquitinating enzymes (DUB), which play an important role in numerous biologically important cellular processes including control of growth, differentiation, oncogenesis and genome integrity [10, 11]. Deubiquitination of macromolecules by DUB, including ubiquitin proteases, can rescue macromolecules from degradation through substrate-specific, N-terminal dependent, enzymatic reaction [12]. During spermatogenesis, these enzymes might be involved in processes such as the removal of histones, regulation of protein turnover during meiosis, germ cell apoptosis, mitotic proliferation and differentiation of spermatogonial stem cells [13–19]. USP26 consists of a single exon and is located on Xq26.2. The mRNA sequence of the USP26 gene is 2,794 bp long and its protein consists of 913 amino acids (Genbank:NM_031907.1). Exclusive expression of the gene in the testis was determined in both mice and humans. Its mouse homolog was found to be only expressed in spermatogonia [9]. Given the importance of DUBs, the testis-specific expression of USP26, its putative role in regulation of the spermatogenesis, and its location on the X chromosome, which limits the gene to a single allele in an individual, this gene have been considered as a novel and attractive candidate gene for the study of male infertility [6, 8, 9].
In average, spontaneous pregnancy loss is a disappointing condition and a common occurrence, with approximately 15 % of all clinically recognized pregnancies resulting in pregnancy failure. Recurrent pregnancy loss (RPL) has been inconsistently defined. When defined as three consecutive pregnancy losses prior to 20 weeks from the last menstrual period, it affects approximately 1 % to 2 % of women of childbearing age [20]. Although some causes have been identified, others remained unclear. Thus, 50 % of couples are still classified as having unexplained RPL, since the underlying mechanisms have not been identified yet [21]. However, most of known causes are related to the female, with the male’s contribution remaining relatively underexplored. We knew from the past that abnormal numbers of chromosomes or abnormal rearrangements of chromosomes in the male parent will result in a higher miscarriage rate, and reduced fertility [22]. In couples with recurrent miscarriage, the incidence of either of the partners being a carrier of a structural chromosome abnormality is ∼3–4 %, mainly consisting of reciprocal translocations (61 %) and Robertsonian translocations (16 %) [23, 24]. It is found that the chromosomes within the spermatoozoan itself can be altered over time due to paternal age, medicinal effects, radiation effects, or environmental factors [25]. Sperm DNA fragmentation has been related to male subfertility, sporadic abortion and poorer reproductive outcome, especially after assisted conception technologies [26–31], but with contradictory results [32–35]. Increased histone levels in sperm DNA after complete differentiation is associated with increased sperm DNA damage [36]. Anything that damages the sperm’s DNA integrity can significantly increase DNA fragmentation which also can result in higher miscarriage rates [21]. Moreover, previous studies have addressed the importance of the ubiquitin pathway during mammalian fertilization, including acrosomal function, spermatozoa–zona pellucida (ZP) penetration, embryo formation and even embryo development [37–39].
Several sequence changes in the USP26 gene were detected in populations of men with severe male factor infertility; including men with Sertoli cell only syndrome (SCO) and maturation arrest [6, 40]. Three mutations, usually found to be clustered in the same allele, were detected in these patients. The cluster mutations were 370–371insACA, 494 T > C and 1423C > T, which led to the amino acid changes T123–124ins, L165S and H475Y, respectively. The coexistence of these three changes in a cluster might point to a specific X chromosome haplotype. Interestingly, in a publication on USP26, the authors identified additional compound mutation that included the 370–371insACA mutation and a new change (460G > A) in the USP26 gene that might cause spermatogenesis impairment in a small group of Chinese infertile oligozoospermic men [1, 40, 41]. Paduch et al. (2005) [40] demonstrated two additional changes, 1090C > T and 1737G > A, with frequencies of 4.3 and 1.6 %, respectively, but they were not investigated in greater depth.
Consequently we proposed, for the first time, the screening of USP26 gene alterations as a work-up tool not only for infertile men with azoospermia, but also for the males of couples with recurrent pregnancy loss (RPL). Accordingly, further studies based on a large group of patients with diversified ethnic backgrounds will be valuable to clarify the role of the observed genetic variants and haplotypes of USP26 in male infertility and in RPL. Therefore, we investigated the five most frequent changes (370–371insACA, 494 T > C, 1423C > T, 1090C > T and 1737G > A) and assessed their frequency in 298 Iranian men with known fertility status (166 men with Azoospermia, 72 male partners of women with idiopathic RPL and 60 fertile men with normal spermatogenesis). Moreover, the relation between the level of USP26 gene expression and spermatogenesis progress and also different form of spermatogenic failures were assessed. We also compared our results with clinical findings.
Materials and methods
Study subjects
The study was approved by the Ethical Committee of Royan reproductive and biomedicine research center. All donors gave their informed written consent prior to participation and completed a written questionnaire to obtain information related to their family history and ethnic. The predominant ethnic background of all groups was Iranian (>95 %). All samples were collected during a three-year period (2010–2012).
Azoospermic men
One hundred sixty six infertile men presenting non-obstructive azoospermia were enrolled. The mean age of the azoospermic men was 36.5 ± 5.82 [25–46] years. Individuals previously diagnosed with any condition or treatments connected with infertility (e.g., cystic fibrosis, Klinefelter syndrome, varicocele, chemotherapy, AZF genes micro deletions, etc.) were not included.
Male partners of women with idiopathic RPL
This group was composed of the male partners of 72 Caucasian couples who had previously experienced ≥3 clinical first trimester (5–14 weeks) spontaneous abortions, with normal karyotypes of both male and female and no autoimmune or endocrine disorders. These couples had not attempted assisted reproduction treatments at the time they were accepted for the study. Only men with normal semen analysis with history of RPL were enrolled. The mean age of the men was 34.38 ± 4.52 [27–43] years.
The patients were evaluated according to the standard protocol. Patients with any identifiable cause of male infertility, including congenital bilateral absence of vas deference (CBAVD), cryptorchidism, varicocele, diabetes mellitus or hypertension, or with history that may affect spermatogenesis (e.g., orchitis, trauma, malignancies, etc.) were excluded from the study group. All of the patients underwent comprehensive characterization, including a detailed history, physical examination, at least two semen analyses, hormonal assays (luteinizing hormone [LH], follicle stimulating hormone [FSH]and testosterone [T]), karyotyping and a molecular test for Y-chromosomal deletions. Chromosome analysis was performed using the GTG method (G-banding by Trypsin-Giemsa technique). Molecular analysis of Y-chromosomal deletions included a combination of nine gene-based primers, as described previously [4].
Female partner of all these patients were normal. Women with idiopathic RPL were defined as women who had had three or more consecutive pregnancy losses. The mean age of these women was 28.46 ± 4.34 [21–36] years. The number of miscarriages ranged from three to seven, and the mean was 3.29 ± 0.9. The women were found to have a normal blood karyotype, a normal uterus by vaginal ultrasonography or hysterosalpingography, normal ovarian function, a normal level of thyroid hormones and fasting blood glucose, negative anti-phospholipid antibodies, absence of activated protein C resistance, normal serum homocystine level, normal plasma protein C and protein S levels and none had polycystic ovary syndrome. In addition, for women with RPL, MTHFR C677T, factor V Leiden G1691A and prothrombin G20210A mutations were analyzed and genetic thrombophilic factors discharged.
Controls
Sixty men with proven fertility were examined as control group. None of the fertile men had a clinical history of varicocele, cryptorchidism or inguinal hernia. All of the control subjects had normal semen analysis, at least one child within 3 years without assisted reproductive technologies and no history of miscarriages. The mean age of control group was 34.5 ± 6.17 [24–46] years. All patients and control subjects were Iranian, and live in different places in Iran.
DNA extraction and mutation analysis
DNA was extracted from peripheral blood lymphocytes using Salting out DNA extraction method [42]. All reactions were optimized to give clean ample quantities of DNA. The 370–371insACA mutation was analyzed by 10 % acrylamide gel, and PCR-SSCP method while the 494 T > C, 1423C > T, 1090C > T and 1737G > A mutations were detected by restriction analysis performed on the PCR products and analyzed on an agarose gel (1.7 %). A mismatch in the forward primer was introduced for distinguishing between the normal allele and the mutant allele on position 1737 by restriction with FokI. The specific restriction enzymes and primers pair for each mutation were selected according to Ribarski et al.; 2009 [41] and are summarized in Table 1. Cloning and sequencing were performed for confirming PCR-SSCP analysis results. Sequencing of PCR products was carried out by Fazabiotech Company (Tehran, Iran) according to Sanger method using ABI 3730XL Capillary Sequencer. Sequencing results were compared with the sequence of normal USP26 gene (NC_000023) obtained from the NCBI website: http://www.ncbi.nlm.nih.gov (Fig. 1).
Table 1.
PCR primers, annealing temperature, product size and fragments sizes after restriction treatment for detection of the USP26 variations
| Mutation | Amino acid change | Primer Sequence | PCR product size | Restriction enzyme | Annealing temp | Restriction fragments (bp) | |
|---|---|---|---|---|---|---|---|
| Mutant Allele | Normal Allele | ||||||
| Ins ACA 370-371a | T 123–124 ins | 5′ GACCTGGTAAGGGTGGGAGT 3′ | 81 bp | – | 60 | – | |
| 5′ TCTCATCAACTTTGTGGAATGAA 3′ | |||||||
| 1423C > Ta | H475Y | 5′ TGTTGCACTCCATTGCTTGT 3′ | 303 bp | FokI | 62 | 303 | 218-85 |
| 5′ TGGAAATGATGACTTCCTGGT 3′ | |||||||
| 494 T > Ca | L165S | 5′ GGAAACCAAAATCACCTGCAT 3′ | 547 bp | TaqI | 62 | 298-138 | 436-91 |
| 5′ AAGGATTTCCAGTGGCGTTC 3′ | 91-20 | 20 | |||||
| 1090C > T | L364F | 5′ TTTCAATCCCATCGTTTGCT 3′ | 400 bp | DraI | 59 | 247-104 | 295-104 |
| 5′ GCAATGGAGTGCAACAACTC 3′ | 48 | ||||||
| 1737G > A | M579I | b5′ CAATTATTAAAAGTTATTCGAAGGAT3′ | 196 bp | FokI | 55 | 196 | 160-36 |
| 5′ TTTGGTTTAGAATTTTTTCCAA 3′ | |||||||
aThe three mutations that were always found in the same allele
bThe mismatch added to the primer is in bold underline
Fig. 1.
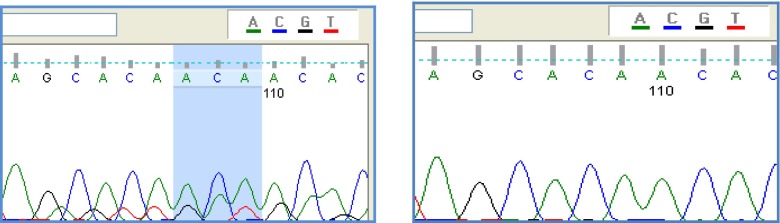
Above figure shows sequence with ACA insertion and the following shows one without any insertion
Tissue samples, mRNA extraction and expression study
Ethical approval and informed patient consent was obtained for the use of tissue samples in this study. In brief, testicular biopsies from 24 azoospermic infertile men were analyzed. The mean age of these men was 33.2 ± 6.1 [23–44] years.
Testicular tissue was obtained from unused portions of multi-site testicular biopsies (TESE) for diagnostic and therapeutic reasons from patients undergoing ICSI treatment at the same time. The tissues were immediately snap-frozen in nitrogen vapor and kept at −80 °C until RNA extraction. In order to examine USP26 mRNA expression pattern in human testis, total RNA was extracted from 24 testicular samples; eight patients with obstructive azoospermia and normal histology, nine patients with Complete Maturation Arrest at Spermatocyte level, and seven patients with Sertoli Cell Only syndrome (SCO). The integrity of total RNA was checked by denaturing formaldehyde/MOPS/1 % agarose electrophoresis and the purity was checked by UV-spectrophotometry in 10 mM Na2HPO4/NaH2PO4-buffer (pH 7.0). The A260/A280-ratio was >2.0. Two distinct ribosomal RNA bands were identified in each sample examined. To amplify USP26 from cDNA, the following set of primers was used:
5′- TCC TTA AAC TAG CAG CAC CA -3′
5′- AAA CTG TAT CAA GCA TCA CGG -3′ (139- bp long product).
GAPDH was used to verify the quality of cDNA synthesis and PCR reaction. The following set of primers was used to amplify GAPDH:
5′- CTC ATT TCC TGG TAT GAC AAC GA -3′
5′- CTT CCT CTT GTG CTC TTG CT -3′
To exclude genomic amplification, PCR was performed with the same total RNA samples without reverse transcriptase. Products were analyzed on 4 % agarose gel. One Step Quantitative RT-PCR was performed by 7500 Real time PCR system (Applied Bio System-USA) on Applied Bio System PCR mastermix in triplicate reaction to ensure consistent. Temperature profile of the real time PCR consists of 95 °C 10 min, 40 cycles of 95 °C 15 s and 60 °C 1 min. REST384-β(2006) software was used to compare means between groups.
Statistical analysis
All statistical analyses were carried out using SPSS (SPSS Inc., Chicago, Illinois, USA) software version 15. All values are expressed as the mean and range or mean ± SD. To analyze the results of the gene variations and mutations between all groups, the χ2- test and Fisher Exact test were used. P-value of less than 0.05 was considered as a significant level. Student’s t-test assessed the significance of testicular volume, FSH, LH and testosterone level between the groups. All statistics were performed at the Epidemiology Department of Royan reproductive biomedicine research center.
Results
Detailed description of identified mutations
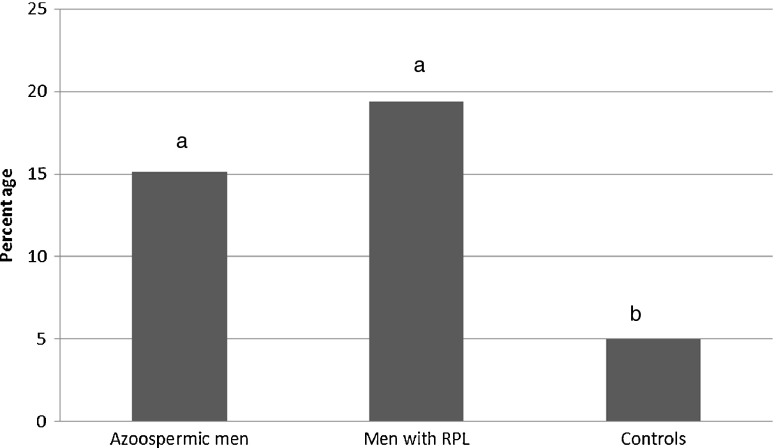
In total, five genetic variants were investigated, including one insertion variant 370-371insACA, causing a threonine insertion in amino acid position 121, and four SNPs, 494 T > C, , 1423C > T, 1737G > A and 1090C > T. Four SNPs were predicted to create amino acid alteration: 494 T > C changes a Leucine into a Serine (L165S); 1423C > T substitutes a Histidine for a Tyrosine (H475Y); and 1737G > A substitutes a Methionine for an Isoleucine (M579I). Totally 25 mutations in azoospermic group (%15.1), 14 mutations in male partners of women with idiopathic RPL (%19.4) and only three mutations in control group (%5.0) in USP26 gene were revealed. The frequency of mutations in azoospermic cases and men with history of idiopathic RPL was significantly higher than that of in control groups (p < 0.05) (Fig. 2).
Fig. 2.
Frequency of different USP26 mutations in the study groups. The same letter show not significant different with P- value >0.05
Table 2 shows the result of haplotype observations. Haplotype analyses released that 370-371insACA and 1423C > T variant sites are always together. Wild type of 494 T in combination with 370-371insACA and 1423C > T was only observed in azoospermic patients (4 men), although, in all groups there are cases who are carrying 494 T > C with above mentioned variant sites too. This haplotype was detected in three azoospermic men with Sertoli cell-only (SCO) syndrome and in one man with maturation arrest. The 1737G > A substitution occurred only in three azoospermic patients but not in the other groups. The 1090C > T substitution occurred twice in azoospermic patients and also twice in men with history of idiopathic RPL but it wasn’t seen in control group. Of the five variants, 1737G > A only was seen exclusively in azoospermic group.
Table 2.
Haplotype frequency in each sample group
| insACA370-371 | 1423C > T | 494 T > C | Controls n (%) | Azoosperm patients n (%) | Men with history of RPL n (%) | P-Value |
|---|---|---|---|---|---|---|
| Yes | T | T | 0 % | 4(2.41 %) | 0 % | 0.401 |
| Yes | T | C | 1(1.67 %) | 4(2.41 %) | 4(5.55 %) | 0.416 |
Allelic frequencies for each sequence variant of USP26 are shown in Table 3.
Table 3.
Result of USP26 mutations survey in the study groups
| insACA370-371 | 494 T > C | 1090C > T | 1423C > T | 1737G > A | Total | P-Value¥ | |
|---|---|---|---|---|---|---|---|
| Control (n = 60) | 1(1.67 %) | 1(1.67 %) | 0 | 1(1.67 %) | 0 | 3(5 %)a | 0.043 |
| Men with history of RPL (n = 72) | 4(5.55 %) | 4(5.55 %) | 2(2.78 %) | 4(5.55 %) | 0 | 14(19.4 %)b | |
| Azoospermic Men (n = 166) | 8(4.81 %) | 4(2.40 %) | 2(1.20 %) | 8(4.81 %) | 3(1.80 %) | 25(15.1 %)b |
a,bThe same letter show not significant difference between groups
¥P-Value for comparing percents of total mutations in the study groups
Phenotypic changes identified in patients with USP26 mutations
No significant differences in the testis volume were observed between infertile men (azoospermic cases and men with history of RPL) with (n = 19; 14.41 ± 7.39 ml) and without (n = 219; 15.1 ± 8.42 ml) the mutations (P = 0.26). The level of testosterone was normal in 82 % of men without mutation while 78 % of cases with mutation had a normal testosterone range. Although the level of testosterone was slightly lower in infertile men (both azoospermic men and men with history of RPL) with mutation than that of in men without mutations, but the difference was not statistically significant. Besides, no significant difference was found between serum FSH and LH between infertile men with and without the mutations. As the mutations were detected only in one man in control group, no comparison of mean serum hormonal levels between men with and without mutation was possible in this group. The results are summarized in Table 4.
Table 4.
Hormonal profile of men with/without mutations in different groups
| Groups | LH(IU/l) | P-Value | FSH(IU/L) | P-Value | Testostrone (nmol/L) | P-Value | |
|---|---|---|---|---|---|---|---|
| Azoospermic men | With Mutation | 8.83 ± 6.32 | 0.22 | 18.38 ± 15.23 | 0.479 | 17.06 ± 12.14 | 0.478 |
| Without Mutation | 6.8 ± 5.39 | 21.69 ± 21.52 | 19.61 ± 11.84 | ||||
| Men with history of RPL | With Mutation | 7.38 ± 6.4 | 0.772 | 19.17 ± 20.66 | 0.895 | 21.32 ± 10.87 | 0.621 |
| Without Mutation | 8.21 ± 6.01 | 17.96 ± 16.51 | 23.81 ± 14.53 | ||||
Testicular tissues from azoospermic patients who underwent TESE were histologically and cytologically evaluated. Accordingly, 56 (33.73 %) men had hypospermatogenesis, 50 (30.12 %) had complete maturation arrest at spermatocyte level and 60 (36.15 %) had SCO. Changes in the USP26 gene were found in 8.33 % (5/60) of patients with Sertoli cell-only syndrome (SCOS) and 8 % (4/50) of patients with complete maturation arrest. Moreover, these modifications were also found in 7.14 % (4/56) patients with hypo spermatogenesis or incomplete maturation arrest. No significant associations were found between the presence of USP26 mutations and the result of testis histological and cytological patterns (p > 0.05).
Expression & real time analysis
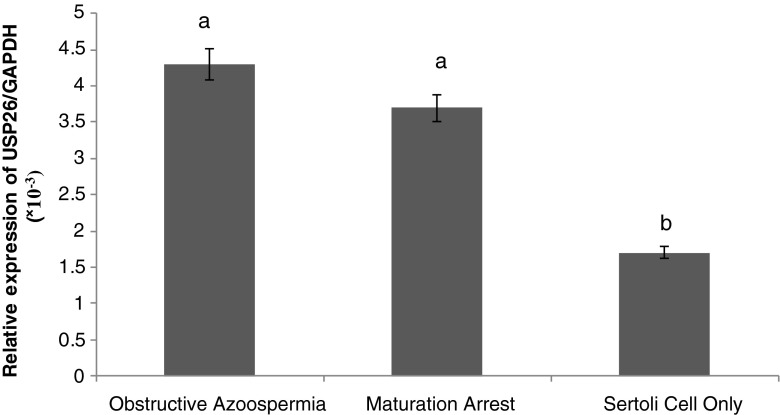
To evaluate the differences of USP26 gene expression in testes of azoospermic patients with different pathological results, quantitative RT-PCR was performed. GAPDH primers were used as internal control. In order to minimize the experimental error, all the stages except RNA extraction were repeated twice. Data analysis were done by 2^-Δct (Fig. 3). The results indicated that USP26 is expressed in testis with normal histology, as well in testes samples with SCO and complete maturation arrest. The USP26 gene expression in infertile men with obstructive azoospermia is higher than that of patients with SCO and complete maturation arrest. The statistical analysis showed that there is a significant difference between the expression levels of USP26 gene in tissues of obstructive azoospermia and complete maturation arrest samples compared to SCO samples (P < 0.05). Although the USP26 gene expression rate in patients with complete maturation arrest was slightly lower than that of in patients with obstructive azoospermia, It was not statistically significant (P > 0.05) (Fig. 3).
Fig. 3.
Result of 2^-Δct of usp26 expression level relative to GAPDH in different groups. The same letter show not significant different with P- value >0.05
Discussion
There are more than 3,000 genes which are involved in the genetic network required for male fertility [43]. Different studies have been mainly focusing on genes with a testis-specific expression pattern. The human X chromosome is enriched with testis-specific genes that may be crucial for male fertility [9]. One of them is USP26 and several papers have described the role of its mutations in male infertility [6, 13, 15, 40, 41]. It is a deubiquitinating enzyme and plays an important role in a wide variety of cellular processes such as the ones described before [13, 44, 45]. Studies in mice have shown the presence of USP26 mRNA throughout all stages of spermatogenesis [9]. Although the expression pattern was firstly reported to be testis-specific in mice and humans [6, 9, 40], it was demonstrated that it can be expressed in human ovary as well, at a level 10-fold lower than in the testis [46]. According to this expression pattern and the importance of ubiquitin and deubiquitinating enzymes in different cellular processes, we supposed that USP26 might have a key function during spermatogenesis. We analyzed azoospermic patients with various histological patterns of spermatogenic defects (Sertoli cell-only syndrome and maturation arrest) for the presence of mutations in USP26. There were no significant associations between the presence of USP26 mutations and the testis histological and cytological patterns. All of the selected sequence changes in the USP26 gene were detected in some infertile azoospermic men (Table 3). In 4 azoospermic patients only 370_371insACA and 1423C > T were observed. Moreover, three mutations (370-371insACA, 494 T > C and 1423C > T), usually found to be clustered in the same allele [1, 40, 41], were detected in 4 azoospermic men. We also found some cases in the other two groups who had the same genotype (Tables 2 and 3). The coexistence of these three changes in a cluster, point to a specific X chromosome haplotype. This combination of changes was also detected in the study of Paduch et al. in four patients. They have found none of these changes in their control group [40]. In contrast to their results, in this study, the combination of 370_371insACA, 494 T > C and 1423C > T were detected in patients with the history of RPL and also in our control group (Tables 2 and 3).
Although the study of Paduch [40] suggested that a specific genetic cluster (370-371insACA, 494 T > C and 1423C > T) might be associated with testicular dysfunction, other studies showed that this genetic cluster was not limited to men with testicular dysfunction [47, 48]. Our results are in concordance with theirs. In the report of Ravel et al. [47], the haplotype was identified in significant frequencies in some populations, including those with known fertility. In another report examining the haplotype frequency, Stouffs et al.[48] examined a large group of Caucasian patients and control subjects and detected the haplotype in a single fertile control, consistent with the findings by Ravel et al.[6, 47]. On the other hand, some of previous case–control studies revealed no significant association of the 370-371insACA, 494 T > C and 1423C > T genotype with male infertility [6, 40, 47–49]. In one report, by Stouffs et al. [48] these changes were identified in 7.2 % of individuals screened with Sertoli cell–only syndrome (SCOS) but in none of the individuals with maturation arrest or with known fertility. The same changes were also identified in another report, by Paduch et al. [40], at a frequency of 9.1 % in patients with a known SCOS histology. We observed this haplotype in 5.55 % of our azoospermic patients, but it was not limited to patients with SCOS. Our results demonstrated that the prevalence of this haplotype was not significantly higher in azoospermic male and men with RPL than that of in control groups (Tables 2 and 3.) so, it cannot be assumed as a direct cause of infertility which can arrests spermatogenesis , because it was observed in all groups.
In addition to focus on just the 370–371insACA, 494 T > C, and 1423C > T haplotype, two other variants in the gene were also evaluated. The 1737G > A substitution occurred only in azoospermic patients. The 1090C > T substitution occurred in azoospermic patients and also in men with history of idiopathic RPL but it wasn’t seen in control group. In our research, the frequency of total mutation was 15.1 % in azoospermic patients (25/166) and 19.4 % of men with idiopathic RPL (14/72) (Table 3). Paduch et al. identified several variants causing an amino acid changes, leading to infertility in a total of 10.6 % of patients (20 of 188) [40]. We concluded that USP26 gene alterations may either cause infertility or be a predisposing factor.
The USP26 gene is expressed in the preliminary stages of spermatogenesis [36, 40]. According to our results, expression of USP26 is not limited to germ cells meanwhile; it is also expressed in Sertoli cells. Our findings on the presence of USP26 mRNA are in agreement with another research which reported USP26 expression in patients with normal histology, SCO, and maturation arrest [40]. To our knowledge, this study is the first report of comparing the expression level of USP26 in different azoospermic patients. No study so far has evaluated expression levels of the USP26 mRNA by Quantitative RT-PCR. Lower expression of USP26 was anticipated in patients with SCO, and this experiment has proved us right. In our study, the expression levels of USP26 gene were lower in patients with SCO than that of in patients with obstructive azoospermia with complete spermatogenesis.
Defective chromatin packing and increased histone to protamine ratio are some of the factors which lead to DNA damage [36, 50, 51]. USP26 is involved in histone removal during protamination consequently its malfunction may lead to DNA damage [40]. A negative association between the degree of DNA damage with various indices of fertility such as fertilization rate, embryo cleavage rate, implantation rate, pregnancy and live birth rate was observed [52]. Sperm DNA damage is also one of the major underlying causes of recurrent spontaneous abortions [50]. The spontaneous abortion rate is 1.7-fold higher when more than 30 % of sperms contain fragmented DNA. The chances of live birth and conception decreases drastically when DNA fragmentation index is >30 [53]. In a research reported by Paduch et al. there were no live deliveries in couples with the USP26 mutation [40]. Mutations and lower expression of USP26 may lead to increase histone levels in sperm DNA after complete differentiation and consequently to increase the sperm DNA damage. Men with idiopathic RPL may have apparently normal sperm parameters, but the germ cells may harbor DNA damages, which cannot be predicted by routine semen analysis [52]. Our result supports important roles of USP26 in male fertility.
Conclusion
In conclusion, the present data suggest that USP26 gene mutations could play crucial role in causing infertility in men and these mutations might be associated with infertility in couples who suffer from RPL. A number of mechanisms have been previously reported to show how the paternal component can affect the embryo, implantation and recurrent miscarriages. This fact more strongly suggests a much more important role for the male factor in the recurrent miscarriage etiologies.
Acknowledgments
We express gratitude to all the participants involved in this study. We acknowledge the efforts of genetic laboratory staff of Royan Reproductive biomedicine research center specially Mrs. Anissi and Mrs Mokhtari.
Declaration of interest
The authors declare that no conflicts of interest exist. This work was supported by Royan Institute for Reproductive biomedicine, ACECR.
Footnotes
Capsule In recent years, a lot of attention has been paid to genetic causes of male infertility. Moreover altered gene expression in spermatogenesis has been showed. Studies are mainly focusing on genes with a testis-specific expression pattern. Such genes which located on the sex chromosomes seem to be more important as men are hemizygous for these chromosomes. We analyzed the Ubiquitin Specific Protease 26 (USP26) gene for the presence of mutations in men with severe fertility problems.
References
- 1.Zhang J, Qiu SD, Li SB, Zhou DX, Tian H, Huo YW, et al. Novel mutations in ubiquitin-specific protease 26 gene might cause spermatogenesis impairment and male infertility. Asian J Androl. 2007;9(6):809–14. doi: 10.1111/j.1745-7262.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 2.Huynh T, Mollard R, Trounson A. Selected genetic factors associated with male infertility. Hum Reprod Update. 2002;8(2):183–98. doi: 10.1093/humupd/8.2.183. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Xiao CY, Zhou-Cun A, Zhang SZ, Li X, Zhang SX. DAZ1/DAZ2 cluster deletion mediated by gr/gr recombination per se may not be sufficient for spermatogenesis impairment: a study of Chinese normozoospermic men. Asian J Androl. 2006;8(2):183–7. doi: 10.1111/j.1745-7262.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 4.Totonchi M, Mohseni Meybodi A, Borjian Boroujeni P, Sedighi Gilani M, Almadani N, Gourabi H. Clinical data for 185 infertile Iranian men with Y-chromosome microdeletion. J Assist Reprod Genet. 2012;29(8):847–53. doi: 10.1007/s10815-012-9798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernando L, Gromoll J, Weerasooriya TR, Nieschlag E, Simoni M. Y-chromosomal microdeletions and partial deletions of the Azoospermia Factor c (AZFc) region in normozoospermic, severe oligozoospermic and azoospermic men in Sri Lanka. Asian J Androl. 2006;8(1):39–44. doi: 10.1111/j.1745-7262.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 6.Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. Possible role of USP26 in patients with severely impaired spermatogenesis. Eur J Hum Genet. 2005;13(3):336–40. doi: 10.1038/sj.ejhg.5201335. [DOI] [PubMed] [Google Scholar]
- 7.Stouffs K, Willems A, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. The role of the testis-specific gene hTAF7L in the aetiology of male infertility. Mol Hum Reprod. 2006;12(4):263–7. doi: 10.1093/molehr/gal020. [DOI] [PubMed] [Google Scholar]
- 8.Nishimune Y, Tanaka H. Infertility caused by polymorphisms or mutations in spermatogenesis-specific genes. J Androl. 2006;27(3):326–34. doi: 10.2164/jandrol.05162. [DOI] [PubMed] [Google Scholar]
- 9.Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27(4):422–6. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 10.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 11.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695(1–3):189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997;11(14):1245–56. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- 13.Lee IW, Kuan LC, Lin CH, Pan HA, Hsu CC, Tsai YC, et al. Association of USP26 haplotypes in men in Taiwan, China with severe spermatogenic defect. Asian J Androl. 2008;10(6):896–904. doi: 10.1111/j.1745-7262.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 14.Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M, et al. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13(5):705–16. doi: 10.1016/j.devcel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Christensen GL, Griffin J, Carrell DT. Sequence analysis of the X-linked USP26 gene in severe male factor infertility patients and fertile controls. Fertil Steril. 2008;90(3):851–2. doi: 10.1016/j.fertnstert.2007.06.096. [DOI] [PubMed] [Google Scholar]
- 16.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123(5):773–86. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Sakai N, Sawada MT, Sawada H. Non-traditional roles of ubiquitin-proteasome system in fertilization and gametogenesis. Int J Biochem Cell Biol. 2004;36(5):776–84. doi: 10.1016/S1357-2725(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 18.Baarends WM, Roest HP, Grootegoed JA. The ubiquitin system in gametogenesis. Mol Cell Endocrinol. 1999;151(1–2):5–16. doi: 10.1016/S0303-7207(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 19.Baarends WM, van der Laan R, Grootegoed JA. Specific aspects of the ubiquitin system in spermatogenesis. J Endocrinol Invest. 2000;23(9):597–604. doi: 10.1007/BF03343782. [DOI] [PubMed] [Google Scholar]
- 20.Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Porter TF, Scott JR. Evidence-based care of recurrent miscarriage. Best Pract Res Clin Obstet Gynaecol. 2005;19(1):85–101. doi: 10.1016/j.bpobgyn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Mozdarani H, Meybodi AM, Zari-Moradi S. A cytogenetic study of couples with recurrent spontaneous abortions and infertile patients with recurrent IVF/ICSI failure. Indian J Hum Genet. 2008;14(1):1–6. doi: 10.4103/0971-6866.42319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franssen MT, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ. 2006;332(7544):759–63. doi: 10.1136/bmj.38735.459144.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franssen MT, Musters AM, van der Veen F, Repping S, Leschot NJ, Bossuyt PM, et al. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: a systematic review. Hum Reprod Update. 2011;17(4):467–75. doi: 10.1093/humupd/dmr011. [DOI] [PubMed] [Google Scholar]
- 25.Bellver J, Meseguer M, Muriel L, Garcia-Herrero S, Barreto MA, Garda AL, et al. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum Reprod. 2010;25(7):1713–21. doi: 10.1093/humrep/deq098. [DOI] [PubMed] [Google Scholar]
- 26.Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, Flamigni C, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21(11):2876–81. doi: 10.1093/humrep/del251. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy C, Ahlering P, Rodriguez H, Levy S, Sutovsky P. Sperm chromatin structure correlates with spontaneous abortion and multiple pregnancy rates in assisted reproduction. Reprod Biomed Online. 2011;22(3):272–6. doi: 10.1016/j.rbmo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Lewis SE, Agbaje I, Alvarez J. Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med. 2008;54(3):111–25. doi: 10.1080/19396360801957739. [DOI] [PubMed] [Google Scholar]
- 29.Lin MH, Kuo-Kuang Lee R, Li SH, Lu CH, Sun FJ, Hwu YM. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril. 2008;90(2):352–9. doi: 10.1016/j.fertnstert.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23(12):2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 31.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57(1–2):78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 32.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89(4):823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 33.Nicopoullos JD, Gilling-Smith C, Almeida PA, Homa S, Norman-Taylor JQ, Ramsay JW. Sperm DNA fragmentation in subfertile men: the effect on the outcome of intracytoplasmic sperm injection and correlation with sperm variables. BJU Int. 2008;101(12):1553–60. doi: 10.1111/j.1464-410X.2008.07518.x. [DOI] [PubMed] [Google Scholar]
- 34.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91(4):1119–26. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 35.Baarends WM, van der Laan R, Grootegoed JA. DNA repair mechanisms and gametogenesis. Reproduction. 2001;121(1):31–9. doi: 10.1530/rep.0.1210031. [DOI] [PubMed] [Google Scholar]
- 36.Shamsi MB, Kumar K, Dada R. Genetic and epigenetic factors: role in male infertility. Indian J Urol. 2011;27(1):110–20. doi: 10.4103/0970-1591.78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bebington C, Doherty FJ, Fleming SD. Ubiquitin and ubiquitin-protein conjugates are present in human cytotrophoblast throughout gestation. Early Pregnancy. 2000;4(4):240–52. [PubMed] [Google Scholar]
- 38.Yi YJ, Manandhar G, Sutovsky M, Li R, Jonakova V, Oko R, et al. Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization. Biol Reprod. 2007;77(5):780–93. doi: 10.1095/biolreprod.107.061275. [DOI] [PubMed] [Google Scholar]
- 39.Yi YJ, Manandhar G, Oko RJ, Breed WG, Sutovsky P. Mechanism of sperm-zona pellucida penetration during mammalian fertilization: 26S proteasome as a candidate egg coat lysin. Soc Reprod Fertil Suppl. 2007;63:385–408. [PubMed] [Google Scholar]
- 40.Paduch DA, Mielnik A, Schlegel PN. Novel mutations in testis-specific ubiquitin protease 26 gene may cause male infertility and hypogonadism. Reprod Biomed Online. 2005;10(6):747–54. doi: 10.1016/S1472-6483(10)61119-4. [DOI] [PubMed] [Google Scholar]
- 41.Ribarski I, Lehavi O, Yogev L, Hauser R, Bar-Shira Maymon B, Botchan A, et al. USP26 gene variations in fertile and infertile men. Hum Reprod. 2009;24(2):477–84. doi: 10.1093/humrep/den374. [DOI] [PubMed] [Google Scholar]
- 42.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt PH. Molecular genetics of human male infertility: from genes to new therapeutic perspectives. Curr Pharm Des. 2004;10(5):471–500. doi: 10.2174/1381612043453261. [DOI] [PubMed] [Google Scholar]
- 44.Zarei-Kheirabadi M, Shayegan Nia E, Tavalaee M, Deemeh MR, Arabi M, Forouzanfar M, et al. Evaluation of ubiquitin and annexin V in sperm population selected based on density gradient centrifugation and zeta potential (DGC-Zeta) J Assist Reprod Genet. 2012;29(4):365–71. doi: 10.1007/s10815-011-9689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyamoto T, Tsujimura A, Miyagawa Y, Koh E, Namiki M, Horikawa M, et al. Single nucleotide polymorphism in the UBR2 gene may be a genetic risk factor for Japanese patients with azoospermia by meiotic arrest. J Assist Reprod Genet. 2011;28(8):743–6. doi: 10.1007/s10815-011-9576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koslowski M, Sahin U, Huber C, Tureci O. The human X chromosome is enriched for germline genes expressed in premeiotic germ cells of both sexes. Hum Mol Genet. 2006;15(15):2392–9. doi: 10.1093/hmg/ddl163. [DOI] [PubMed] [Google Scholar]
- 47.Ravel C, El Houate B, Chantot S, Lourenco D, Dumaine A, Rouba H, et al. Haplotypes, mutations and male fertility: the story of the testis-specific ubiquitin protease USP26. Mol Hum Reprod. 2006;12(10):643–6. doi: 10.1093/molehr/gal063. [DOI] [PubMed] [Google Scholar]
- 48.Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. Alterations of the USP26 gene in Caucasian men. Int J Androl. 2006;29(6):614–7. doi: 10.1111/j.1365-2605.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 49.Tuttelmann F, Rajpert-De Meyts E, Nieschlag E, Simoni M. Gene polymorphisms and male infertility—a meta-analysis and literature review. Reprod Biomed Online. 2007;15(6):643–58. doi: 10.1016/S1472-6483(10)60531-7. [DOI] [PubMed] [Google Scholar]
- 50.Shamsi MB, Venkatesh S, Tanwar M, Singh G, Mukherjee S, Malhotra N, et al. Comet assay: a prognostic tool for DNA integrity assessment in infertile men opting for assisted reproduction. Indian J Med Res. 2010;131:675–81. [PubMed] [Google Scholar]
- 51.Shamsi MB, Kumar R, Dada R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J Med Res. 2008;127(2):115–23. [PubMed] [Google Scholar]
- 52.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9(4):331–45. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 53.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]