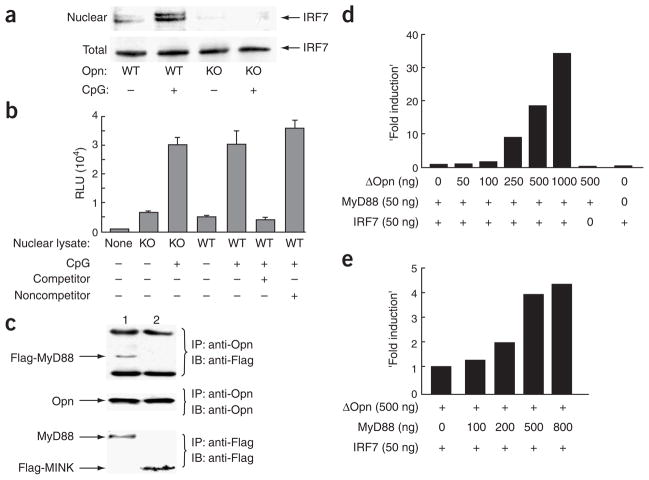
Figure 4.
Biochemical analysis of Opn-i. (a) Nuclear translocation of IRF7 in total bone marrow–derived Opn wild-type (WT) and Opn-deficient (KO) DCs treated with CpG-B (0.4 μM) for 3 h before extraction of nuclear and total lysates. (b) Activation of NF-κB in total bone marrow–derived DCs stimulated for 1 h with 0.3 μM CpG-B. Control competitor and noncompetitor oligonucleotides were added to some samples (+, below graph) to confirm sequence-specific binding of NF-κB in lysate samples. (c) Immunoblot (IB) of protein complexes immunoprecipitated (IP) from HEK293 cells cotransfected for 48 h with Opn, Flag-MyD88 and TLR9 (lane 1) or Opn, Flag-Mink (serine kinase) and TLR2 (lane 2; negative control) followed by stimulation for 3 h with 0.3 μM CpG-B. (d) Opn expression and Ifna4 activation in HEK293 cells transiently transfected with mutant Opn–expressing lentivirus (concentrations, below graph) along with IRF7 and/or MyD88 cDNA. (e) MyD88-dependent Ifna4 activation after transfection of MyD88 expression vector (concentrations, below graph) into HEK293 cells along with mutant Opn and/or IRF7. Data are representative of at least three independent experiments.