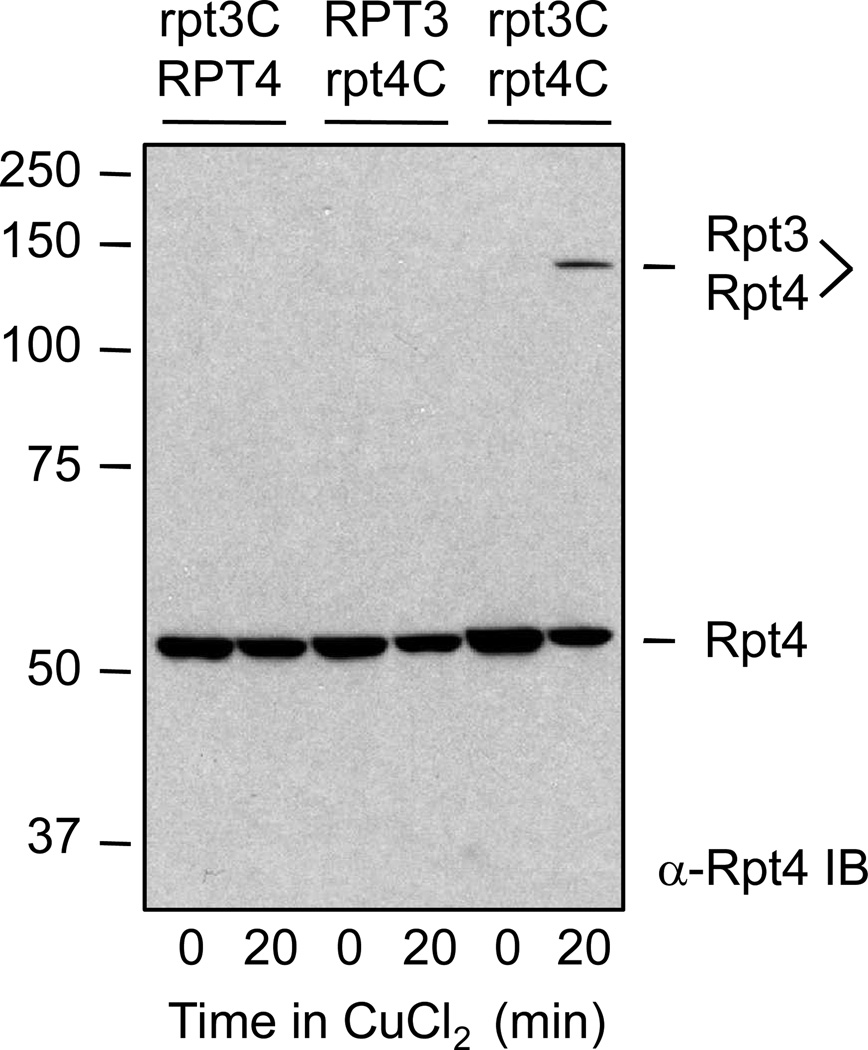
Fig 1.
An example of engineered disulfide crosslinking between two predicted neighboring subunits in the ATPase ring of the S. cerevisiae proteasomal RP. Amino acids were chosen for substitution with cysteine based on primary sequence alignment with the archaeal proteasomal ATPase PAN. The crystal structure of PAN, in turn, suggested residues at the interface between neighboring subunit OB domains that, if replaced with cysteines, could potentially be linked by a disulfide bond. The yeast strains from which whole-cell extracts were made had either an rpt3-I96C or rpt4-R126C mutation or both. Only when both cysteine substitutions were present was a 0.2 mM CuCl2-induced disulfide crosslink observed between the Rpt3 and Rpt4 subunits (last lane). Modified from Ref. 5 (with permission from Cell Press).