Abstract
Objective
To retrospectively evaluate the risk of thyroid cancer in patients with hyperfunctioning thyroid nodules through ultrasonographic-pathologic analysis.
Materials and Methods
Institutional review board approval was obtained and informed consent was waived. From 2003 to 2007, 107 patients consecutively presented with hot spots on thyroid scans and low serum thyroid-stimulating hormone levels. Among them, 32 patients who had undergone thyroid ultrasonography were analyzed in this study. Thyroid nodules depicted on ultrasonography were classified based on size and categorized as benign, indeterminate, or suspicious malignant nodules according to ultrasonographic findings. The thyroid nodules were determined as either hyperfunctioning or coexisting nodules and were then correlated with pathologic results.
Results
In 32 patients, 42 hyperfunctioning nodules (mean number per patient, 1.31; range, 1-6) were observed on thyroid scans and 68 coexisting nodules (mean, 2.13; range, 0-7) were observed on ultrasonography. Twenty-five patients (78.1%) had at least one hyperfunctioning (n = 17, 53.1%) or coexisting (n = 16, 50.0%) nodule that showed a suspicious malignant feature larger than 5 mm (n = 8, 25.0%), or an indeterminate feature 1 cm or greater (n = 20, 62.5%) in diameter, which could have been indicated by using fine needle aspiration (FNA). Seven patients were proven to have 11 thyroid cancers in 3 hyperfunctioning and 8 coexisting nodules. All of these had at least one thyroid cancer, which could have been indicated by using FNA. The estimated minimal risk of thyroid cancer was 6.5% (7/107).
Conclusion
Patients with hyperfunctioning nodules may not be safe from thyroid cancer because hyperfunctioning nodules can coexist with thyroid cancer nodules. To screen out these cancers, ultrasonography should be performed.
Keywords: Hyperfunctioning nodule, Radionuclide imaging, Thyroid cancer, Ultrasonography, Guideline
INTRODUCTION
A hyperfunctioning or "hot" nodule is defined as a nodular region of the thyroid gland, which is visualized as a "hot spot" on thyroid scans due to the larger amount of radiotracer, compared with the surrounding normal thyroid glands (1, 2).
Traditionally, hyperfunctioning thyroid nodules have been known to be exclusively benign lesions such as follicular adenoma (1-6). For this reason, several major current guidelines for thyroid nodules and cancers such as the American Thyroid Association (ATA) guidelines and National Comprehensive Cancer Network Clinical Practice guidelines have generally recommended the avoidance of ultrasonography or fine-needle aspiration (FNA), in favor of evaluating and treating for thyrotoxicosis when a thyroid scan shows a hot spot in patients with low serum thyroid-stimulating hormone (TSH) level (1, 3).
However, with the advent of high-resolution ultrasonography, we have found in our routine clinical practice that there were often unexpected occurrences of coexisting nodules in patients with certain palpable thyroid nodules. Thus, the previously-held belief that hyperfunctioning nodules may safely rule out thyroid cancers may not be applied to patients with hyperfunctioning thyroid nodules as easily as current guidelines suggest. Indeed, upon review of the literature, we have found several reports in which thyroid cancers have occurred in patients with hyperfunctioning nodules or even in the hyperfunctioning nodule itself (7-12). In fact, a few studies have already reported on the frequency of thyroid cancers in patients with hyperfunctioning nodules (9-12). However, until now, it has not been certain that the hot spots seen on the thyroid scans were actually the same nodules determined by pathology, as no studies have been carried out that are based on detailed anatomical imaging such as ultrasonography.
Therefore, in this study, through ultrasonographic-pathologic analysis we retrospectively evaluated whether patients with hyperfunctioning thyroid nodules are really safe from thyroid cancer.
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this retrospective study, and informed consent was waived.
Patients
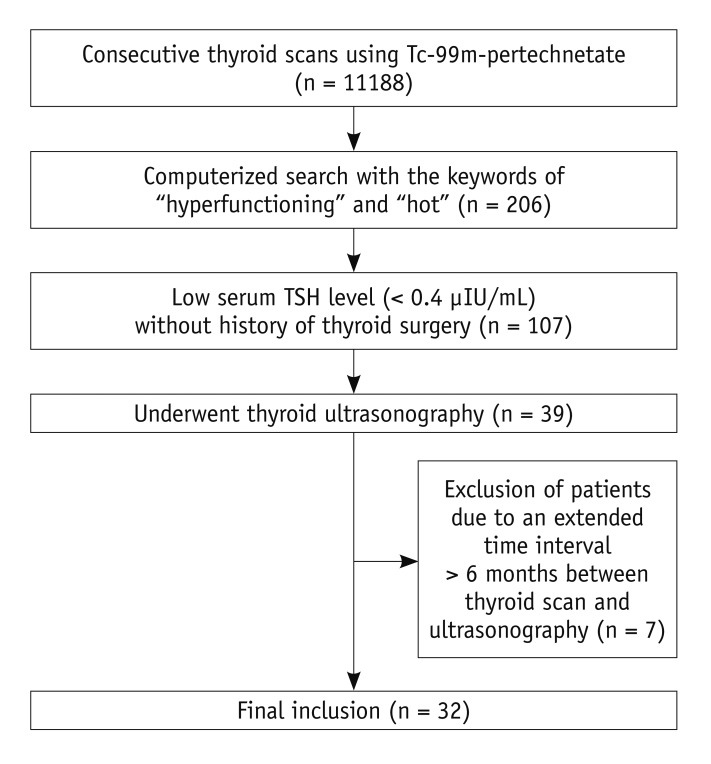
From January 2003 through December 2007, the electronic medical records of our hospital revealed that 11188 consecutive thyroid scans were performed using Tc-99m-pertechnetate at our institution. Using the keywords of "hyperfunctioning" and "hot" on a computerized search of the medical records, one author found that 206 patients were reported to have hyperfunctioning nodules. Among them, 107 patients showed a low serum TSH level (< 0.4 µIU/mL) without a history of thyroid surgery, and 39 patients of those patients had undergone ultrasonography. Finally, after the exclusion of 7 patients due to an extended time interval of more than 6 months between the thyroid scan and ultrasonography, 32 patients (F : M = 29 : 3; mean age, 53.4 years; age range, 29-76 years) comprised our study population (Fig. 1). The mean time interval from the TSH level test to thyroid scan was 5.6 days (median, 1 day; range of difference, - 11 to 87 days) and the mean time interval from the thyroid scan to thyroid ultrasonography was 47.2 days (median, 25 days; range of difference, -86 to 175 days). At the time of thyroid scanning, 10 out of 32 patients presented with thyrotoxicosis.
Fig. 1.
Flowchart of patient selection. TSH = thyroid-stimulating hormone
Thyroid Imaging
All ultrasonographic images were obtained using an Accuvix XQ (Medison Co., Ltd., Seoul, Korea) or an HDI 5000 system (Philips Medical Systems, Bothell, WA, USA) equipped with 7.5 to 12 or 8 to 15 MHz linear transducers. The length, width, and depth of each nodule were measured at their longest aspect on ultrasonographic images which were saved as Digital Imaging and Communications in Medicine images. Faculty radiologists specializing in thyroid imaging (8 and 13 years of experience) either performed the examination or supervised board-certified radiologists who were participating in thyroid radiology fellowship training.
No patient was on anti-thyroid medication at the time of thyroid scanning. Using a single-head gamma camera (LEM Plus, Siemens, Erlangen, Germany) equipped with a pinhole collimator, thyroid scans of the anterior view were obtained 20 minutes after an intravenous administration of 185 MBq of Tc-99m-pertechnetate. For imaging, patients were placed in the supine position with their neck extended, and marking sources for size-measurements were located at both lateral sides of the neck. Oblique views were obtained whenever anterior images could not be adequately evaluated.
Pathologic Examinations
In 24 of the 32 patients, pathologic results were obtained by FNA in 17 patients and by both FNA and surgical histology in 7 patients.
Cytologic material obtained by FNA under ultrasonographic guidance was smeared on slides immediately after aspiration (solid lesion) or after cytologic centrifugation (cystic lesion), and was processed using May-Grunwald Giemsa and Papanicolaou stains. Surgical samples were sectioned before fixation for gross examination and then fixed in 10% buffered formalin, embedded in paraffin, and stained with hematoxylin-eosin for histologic examination.
A pathologist specializing in thyroid pathology (9 years experience) retrospectively reviewed the cytohistologic slides of thyroid nodules without information of their final surgical diagnosis, and determined the pathologic diagnosis using the Bethesda classification for cytologic analysis (13).
Image Analysis
All ultrasonographic images were retrospectively reviewed in consensus by two radiologists who were blinded to the clinical history and pathologic results.
Referring to the Korean Society of Thyroid Radiology (KSThR) recommendations and Korean Thyroid Association (KTA) guidelines (14, 15), thyroid nodules depicted on ultrasonography were categorized into a three-tiered classification of probably benign, indeterminate, and suspicious malignant nodules. The nodules were then classified as less than 5 mm, 5 mm to 1 cm, or larger than 1 cm in maximal dimension.
We then determined which nodules met the ultrasonographic criteria for FNA according to the KSThR recommendations and KTA guidelines (14, 15).
In addition, referring to the ATA guidelines, we evaluated whether the patients had a high-risk history through a review of the medical records and whether ultrasonographic images showed abnormal cervical lymph nodes (1).
Thereafter, a radiologist and a nuclear medicine physician (8 years experience in nuclear medicine) determined in consensus whether the thyroid nodules were hyperfunctioning or coexisting nodules based on the correlation between ultrasonography and thyroid scans. Hyperfunctioning nodules were determined when there was an area where the tracer concentration was greater than the remaining thyroid tissue, or when hemilobar uptake was observed.
Statistical Analysis
All statistical analyses were performed with a commercially available software package (SPSS, version 16.0 for Windows; SPSS Inc., Chicago, IL, USA). Continuous variables were compared using unpaired Student t tests, and categorical variables were compared using chi-square test or Fisher's exact test, as appropriate. A p-value < 0.05 was considered to indicate statistical significance.
RESULTS
Ultrasonographic and Pathologic Results of Hyperfunctioning Nodules and Coexisting Nodules
Thirty-two patients were found to have 42 hyperfunctioning nodules (mean number of patients, 1.31; range 1-6) on thyroid scans and 68 coexisting nodules (mean number of patients, 2.13; range 0-7) on ultrasonography. Twenty-eight of the 32 patients (87.5%) had at least one coexisting nodule. The ultrasonographic and pathologic results of the hyperfunctioning and coexisting nodules are shown in Tables 1, 2.
Table 1.
Ultrasonographic-Pathologic Results of Hyperfunctioning Thyroid Nodules in 32 Patients
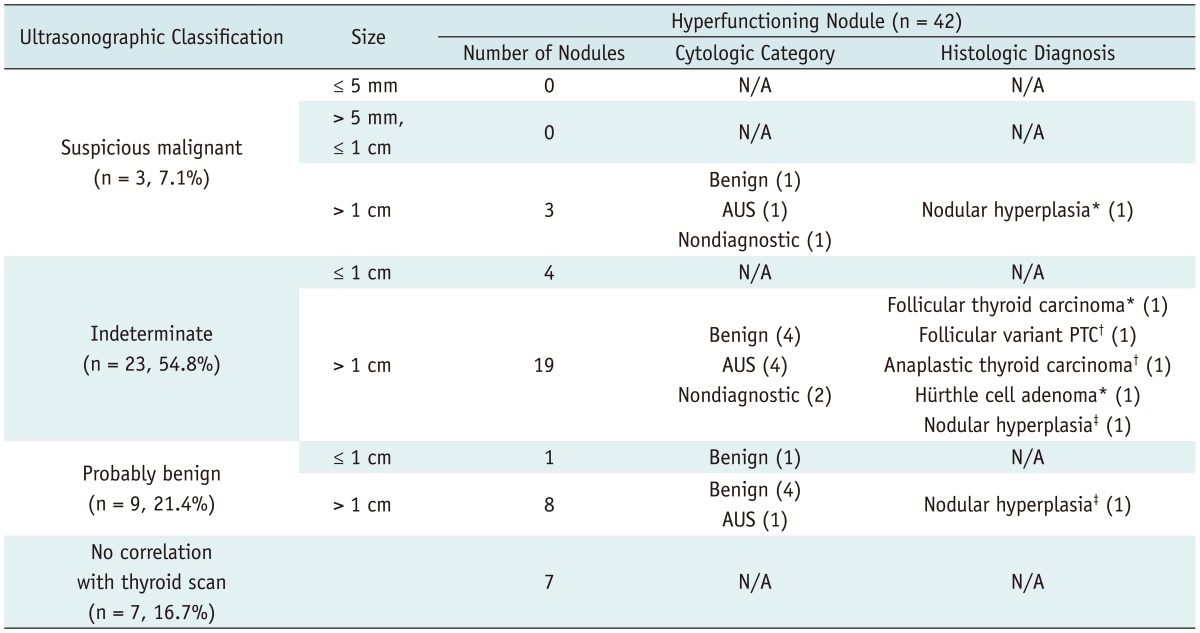
Note.- Numbers in parentheses are numbers of nodules showing individual cytologic or histologic results. *Prior cytology revealed nondiagnostic category, †Prior cytology revealed Atypia of undetermined significance category, ‡Prior cytology revealed benign category. AUS = atypia of undetermined significance, N/A = not applicable
Table 2.
Ultrasonographic-Pathologic Results of Coexisting Thyroid Nodules in 28 of 32 Patients
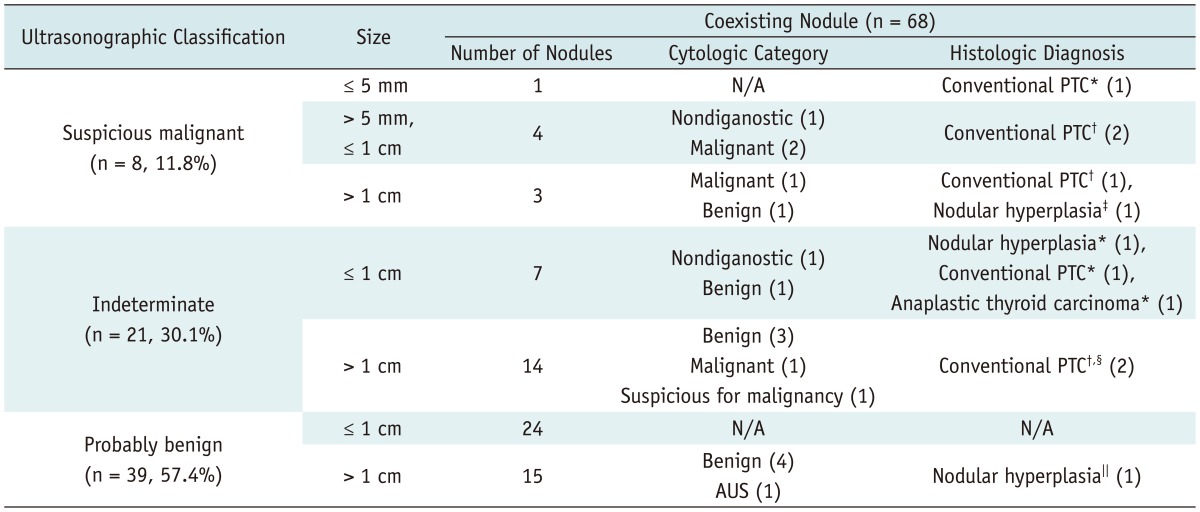
Note.- Numbers in parenthesis are numbers of nodules showing individual cytologic or histologic results. *Prior cytology was not obtained, †Prior cytology revealed malignant category, ‡Prior cytology revealed benign category, §Prior cytology revealed suspicious for malignancy category, ∥Prior cytology revealed atypia of undetermined significance category. AUS = atypia of undetermined significance, N/A = not applicable, PTC = papillary thyroid cancer
Indeterminate features were more frequent in hyperfunctioning nodules than in coexisting nodules (p = 0.0014) and the probably benign feature was more frequent in coexisting nodules than in hyperfunctioning nodules (p = 0.0033). The size of hyperfunctioning nodules (mean, 2.1 cm; range, 0.7-4.4 cm) depicted on ultrasonography was larger than that of coexisting nodules (mean, 1.1 cm; range, 0.3-5.0 cm) (p < 0.0001).
No patient in our study had a high-risk history. One patient who showed abnormal cervical lymph nodes on ultrasonography also showed a thyroid nodule, which could have been indicated with FNA based on our guidelines due to an indeterminate feature larger than 1 cm. A thyroid cancer with lymph node metastasis was proven with both FNA and surgery on the patient (Table 3, patient 7).
Table 3.
Clinical-Ultrasonographic-Pathologic Results of 7 Patients with Thyroid Cancer
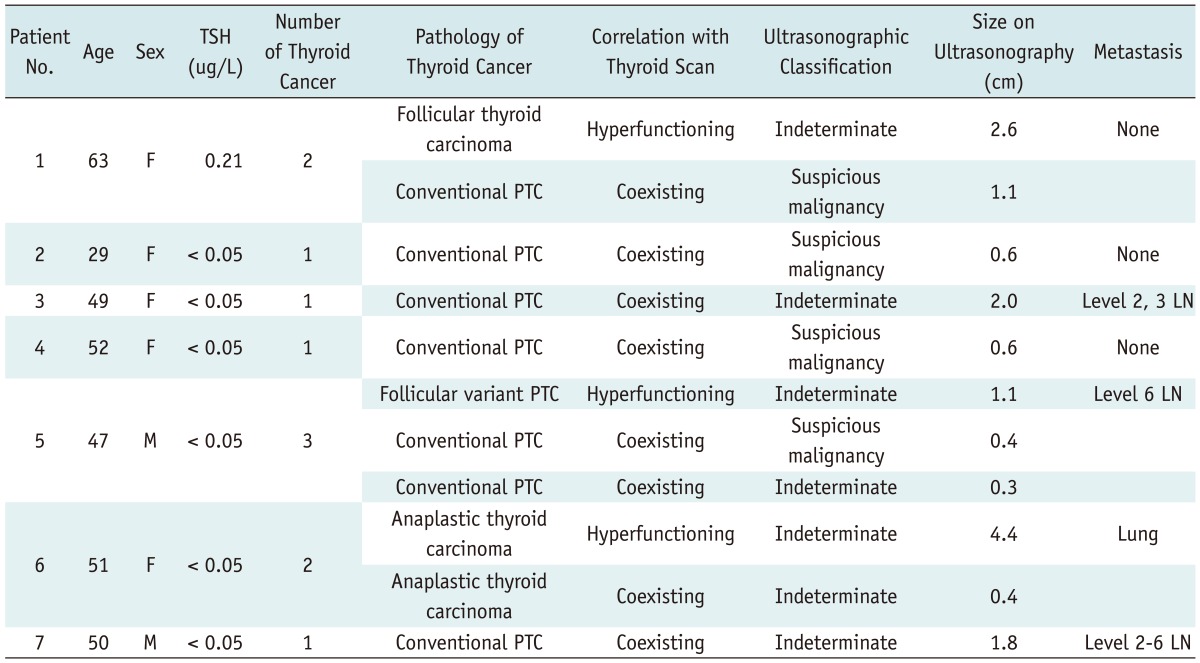
Note.- LN = lymph node, PTC = conventional papillary thyroid carcinoma, TSH = thyroid-stimulating hormone
Twenty-two of the 42 hyperfunctioning nodules (52.4%) in 17 patients (17/32, 53.1%) and 21 of the 68 coexisting nodules (30.1%) in 17 patients (17/32, 53.1%) could have been indicated with FNA. Of the 32 patients, 25 patients (78.1%) had at least one thyroid nodule that showed suspicious malignant features larger than 5 mm (n = 8, 25.0%) and/or indeterminate features 1 cm or greater in size (n = 20, 62.5%), and could have been indicated with FNA.
On cytologic examination, of the 19 hyperfunctioning nodules for which cytologic results were obtained, 10 (52.6%) were reported as "benign", 6 (31.6%) as atypia of unknown clinical significance or follicular lesions with undetermined clinical significance (AUS), and 3 (15.8%) as nondiagnostic. Of the 7 hyperfunctioning nodules, 3 nodules were surgically proven to be thyroid cancers, 3 as nodular hyperplasias, and 1 as Hürthle cell adenoma. Of the 17 coexisting nodules for which cytologic results were obtained, 9 (52.9%) were reported as "benign" on cytology, 4 (23.5%) as malignant, 1 (5.9%) as suspicious for malignancy, 1 (5.9%) as AUS, and 2 (11.8%) as nondiagnostic. Among these 17 coexisting nodules, 9 nodules were surgically proven to be 6 thyroid cancers and 3 nodular hyperplasias. In addition, 2 nodules were surgically proven to be thyroid cancers without previous FNA.
Thyroid Cancer in Patients with Hyperfunctioning Nodules
Table 3 describes the ultrasonographic-pathologic features of thyroid cancers, which occurred in patients with hyperfunctioning nodules. Figures 2-4 show representative cases of hyperfunctioning and coexisting cancers. Of the 32 patients who underwent ultrasonography, 7 patients (21.9%; F : M = 5 : 2; mean age, 49 years; range, 29-63 years) were proven to have 11 thyroid cancers (mean, 2; range 1-3), of which 3 were in hyperfunctioning nodules (1 anaplastic thyroid carcinoma, 1 follicular variant papillary thyroid carcinoma, 1 follicular thyroid carcinoma) and 8 were in coexisting nodules (7 conventional papillary thyroid carcinomas, 1 anaplastic thyroid carcinoma). Three of these 7 patients (42.9%) with thyroid cancers had multiple thyroid cancers. When we estimated minimal cancer risk with a numerator of 7 patients that had thyroid cancers and a denominator of all 107 patients who had hyperfunctioning nodules, whether ultrasonography was performed or not, the minimal cancer risk was 6.5%.
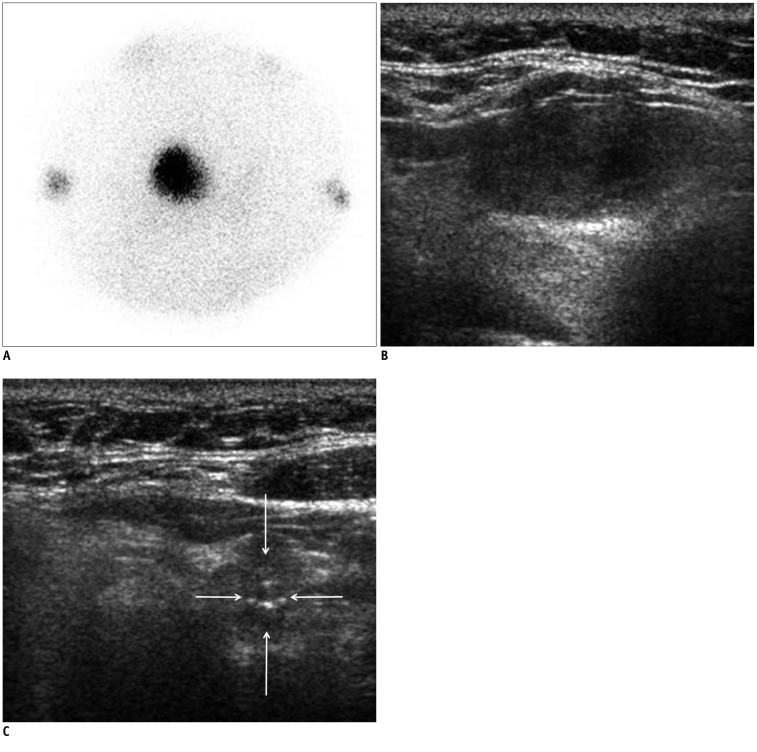
Fig. 2.
Hyperfunctioning cancer and coexisting cancer in 63-year-old woman (Patient No. 1).
A. Thyroid scan shows hot focus on right thyroid gland with suppressed surrounding normal tissue. B. On ultrasonography, low echoic, ovoid to round, smoothly marginated, solid nodule exists without any micro- or macro-calcification in right thyroid gland. It was categorized as indeterminate nodule of 2.6 cm maximal dimension, and could have been indicated with fine needle aspiration. It was surgically proven to be follicular thyroid cancer. C. On ultrasonography, low echoic, taller than wide, spiculated, solid nodule was observed with microcalcification in left thyroid gland (arrows). It was classified as suspicious malignant nodule of 1.1 cm maximal dimension, and could have been indicated for fine needle aspiration. It was surgically proven to be conventional papillary thyroid cancer.
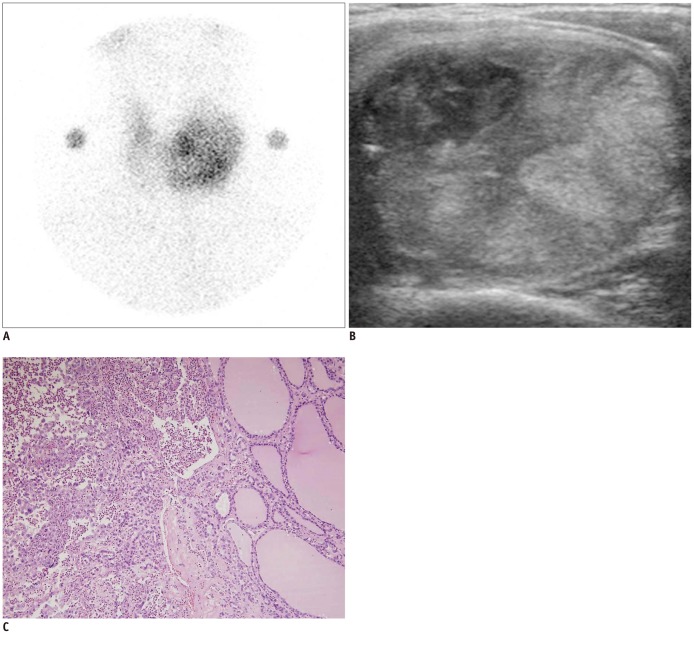
Fig. 4.
Bilateral anaplastic cancers in 51-year-old woman (Pt. No. 6).
A. Thyroid scan shows large heterogeneous hyperfunctioning nodule in left lobe of thyroid gland. B. On ultrasonography, heterogeneous isoechoic, round, smoothly marginated, solid nodule is observed at left thyroid gland. It was classified as suspicious malignant nodule of 4.4 cm maximal dimension, and was indicated with fine needle aspiration. It was surgically proven to be anaplastic thyroid cancer. 0.4 cm indeterminate nodule at right thyroid gland was also proven to be anaplastic carcinoma (not shown). C. 1.8 × 1.8 cm anaplastic thyroid carcinoma is seen under background of follicular carcinoma in left lobe. Follicular carcinoma on right side (neoplastic follicles) transits to anaplastic carcinoma on left side (× 100, H&E).
On ultrasonography, coexisting cancers were equally classified as indeterminate nodules (4/8, 50%) and suspicious malignant nodules (4/8, 50%). In contrast, all 3 hyperfunctioning thyroid cancers were categorized as indeterminate nodules. Although statistical significance was not obtained, the size of hyperfunctioning cancers (mean, 2.7 cm; range, 1.1-4.4 cm) was larger than that of coexisting cancers (mean, 0.9 cm; range, 0.3-2.0 cm) (p = 0.0659).
Four of the 7 patients (57.1%) with thyroid cancers had metastasis on lymph nodes or the lung. Most thyroid cancers (8/11, 72.7%) could have been indicated for FNA: suspicious malignant nodules larger than 5 mm (3/11, 27.3%) or indeterminate nodules 1 cm or greater in size (5/11, 45.5%).
DISCUSSION
Many current guidelines and articles state that if a lesion shows indeterminate cytology such as a follicular neoplasm or a follicular lesion of undetermined significance, the detection of a hyperfunctioning nodule on thyroid scans may prove its benignancy (1-3, 5, 6). However, our study demonstrates that thyroid cancers can indeed develop in the hyperfunctioning nodule itself as well as in coexisting nodules of patients with hyperfunctioning thyroid nodules with an estimated minimal cancer risk of 6.5%. This cancer risk is much higher than the results of ultrasonographic screening in a normal healthy population (16, 17). This is within the range of 5-15% in which thyroid cancer has been known to occur in thyroid nodules, suggesting that the cancer risk in patients with hyperfunctioning nodules may be similar to those who have thyroid nodules other than hyperfunctioning nodules (1, 2). According to the literature, the frequency of malignancy in hyperfunctioning nodules has been reported as up to 11% (9-12). We therefore suggest that patients with hyperfunctioning nodules may not be safe from thyroid cancer.
In terms of the pathologic results of hyperfunctioning nodules in the present study, a benign result was most common upon cytology and thyroid cancer and nodular hyperplasia were most common on histology, which is also somewhat contrary to the belief that follicular adenoma is the most common pathology in hyperfunctioning nodules. Further pathologic studies need to be performed to verify whether the most common pathologic entity is indeed follicular adenoma. According to the literature, the most common hyperfunctioning cancer is follicular thyroid cancer, followed by follicular variant papillary thyroid cancer and conventional papillary thyroid carcinoma, and is consistent with our results (7, 8, 18). We also found that anaplastic cancer may present as a hyperfunctioning nodule, although it was thought to develop in follicular thyroid carcinoma. In addition, over half of the cancer patients in the present study had lymph node or distant metastasis. This concurs with a previous study which reported that metastatic hyperfunctioning thyroid carcinoma was more common than non-metastatic carcinoma (9). Therefore, we believe that we cannot overlook the possibility of thyroid cancer in patients with hyperfunctioning nodules.
Our study showed that 87.5% of patients had coexisting thyroid nodules. This figure is even higher than in Tan et al.'s (19) report in which ultrasonography showed other nodules in 48% of patients with a clinically solitary thyroid nodule. Furthermore, in the present study, 53.1% of patients had at least one coexisting nodule which could have been indicated with FNA, which also concurs with the results by Marqusee et al. (20) who reported that ultrasonography altered the clinical management decision in 63% of cases among patients who had been referred for evaluation of a palpable thyroid abnormality. Current major guidelines reflect these results, as the necessity of thyroid ultrasonography is also being emphasized in patients with a suspected thyroid nodule (1, 2). However, this contradicts another current doctrine that suggests the avoidance of ultrasonography for hyperfunctioning nodules (1, 3).
In addition, we found that both hyperfunctioning benign nodules and cancers have a tendency to be larger in size than coexisting benign nodules and cancers. Furthermore, in the present study, hyperfunctioning cancers showed more frequent indeterminate utrasonographic features than coexisting cancers, which concurs with previous ultrasonographic reports on follicular thyroid cancers and follicular variant thyroid cancers that are common pathologic entities of hyperfunctioning cancer (21-23).
In this study, most patients with hyperfunctioning nodules, who had been proven to have hyperfunctioning and/or coexisting thyroid cancers, could have been screened out in ultrasonography as they had at least one cancer that could have been indicated with FNA; a suspicious malignant feature larger than 5 mm (3/7, 42.9%) and/or an indeterminate feature larger than 1 cm (5/7, 71.4%). If we had used a 1 cm size criteria for suspicious nodules as dictated by ATA guidelines, 7 out of 9 patients (77.8%) could have been screened out in ultrasonography (1). These results demonstrate that it is important to evaluate coexisting nodules as well as the hyperfunctioning nodule itself using high resolution ultrasonography, as ultrasonography can provide both information of multiplicity and detailed characteristics of thyroid nodules that cannot be evaluated through a thyroid scan due to the limited resolution. Although the criteria and suspicious findings for FNA of thyroid nodules slightly differed among the many guidelines, for the most part, we believe that the major guidelines have a similar role for the screening of these patients as the guidelines in the present study (1-3, 14, 15).
Our study has several limitations. First, as a retrospective study with unavoidable selection bias, ultrasonographic and pathologic studies were performed without any consistent principle in patients with hyperfunctioning nodules. Second, the exclusive use of technetium-99m pertechnetate other than radioactive iodine might have caused false-positive results due to trapping without organification (24). However, this might not be a major limitation as the discordant rate between the two examinations was about 1% (25). In addition, the prevalence of hyperfunctioning nodules was significantly higher in iodine-deficient areas (26). As Korea has been classified as an iodine-sufficient area due to our iodine-rich diet, the results of our study should be tested with further prospective studies in iodine deficient areas as well as in other iodine sufficient areas.
In conclusion, patients with hyperfunctioning thyroid nodules may not be safe from thyroid cancer as thyroid cancers can develop in both hyperfunctioning and coexisting nodules at an non-negligible rate, and thus, in order to screen out thyroid cancer, thyroid ultrasonography should be performed in patients with hyperfunctioning thyroid nodules.
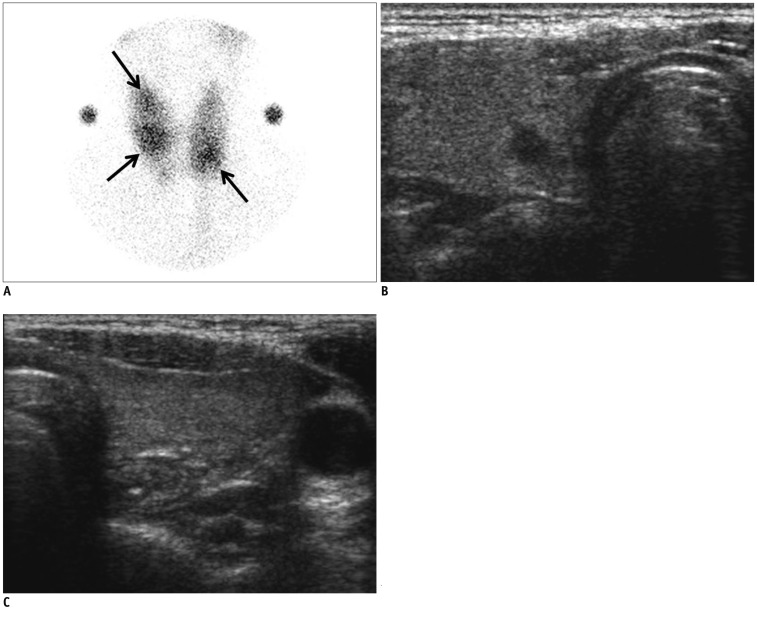
Fig. 3.
Coexisting cancer in 29-year-old woman (Patient No. 2).
A. On thyroid scan, 3 poorly defined hot foci were observed in both thyroid glands (arrows). B, C. On ultrasonography, no nodules which were correlated with thyroid scan could be seen at both thyroid glands. Instead, at right thyroid gland, low echoic, irregular, spiculated, solid nodule without any calcification was noted. It was classified as suspicious malignant nodule of 0.6 cm maximal dimension, and could have been indicated with fine needle aspiration. It was surgically proven to be conventional papillary thyroid cancer.
Acknowledgments
We thank Chris Woo for his editorial assistance.
Footnotes
This study was supported by the research fund of the Radiological Research Foundation of Korea (2009-01).
References
- 1.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, authors. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16(Suppl 1):1–43. doi: 10.4158/10024.GL. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 4.Smith JR, Oates E. Radionuclide imaging of the thyroid gland: patterns, pearls, and pitfalls. Clin Nucl Med. 2004;29:181–193. doi: 10.1097/01.rlu.0000114530.12565.5b. [DOI] [PubMed] [Google Scholar]
- 5.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 6.Mandel SJ. A 64-year-old woman with a thyroid nodule. JAMA. 2004;292:2632–2642. doi: 10.1001/jama.292.21.2632. [DOI] [PubMed] [Google Scholar]
- 7.Harach HR, Sánchez SS, Williams ED. Pathology of the autonomously functioning (hot) thyroid nodule. Ann Diagn Pathol. 2002;6:10–19. doi: 10.1053/adpa.2002.30605. [DOI] [PubMed] [Google Scholar]
- 8.Als C, Gedeon P, Rösler H, Minder C, Netzer P, Laissue JA. Survival analysis of 19 patients with toxic thyroid carcinoma. J Clin Endocrinol Metab. 2002;87:4122–4127. doi: 10.1210/jc.2001-011147. [DOI] [PubMed] [Google Scholar]
- 9.Majima T, Doi K, Komatsu Y, Itoh H, Fukao A, Shigemoto M, et al. Papillary thyroid carcinoma without metastases manifesting as an autonomously functioning thyroid nodule. Endocr J. 2005;52:309–316. doi: 10.1507/endocrj.52.309. [DOI] [PubMed] [Google Scholar]
- 10.Tfayli HM, Teot LA, Indyk JA, Witchel SF. Papillary thyroid carcinoma in an autonomous hyperfunctioning thyroid nodule: case report and review of the literature. Thyroid. 2010;20:1029–1032. doi: 10.1089/thy.2010.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata M, Kasagi K, Hatabu H, Misaki T, Iida Y, Fujita T, et al. Causes of appearance of scintigraphic hot areas on thyroid scintigraphy analyzed with clinical features and comparative ultrasonographic findings. Ann Nucl Med. 2002;16:279–287. doi: 10.1007/BF03000108. [DOI] [PubMed] [Google Scholar]
- 12.David E, Rosen IB, Bain J, James J, Kirsh JC. Management of the hot thyroid nodule. Am J Surg. 1995;170:481–483. doi: 10.1016/s0002-9610(99)80334-1. [DOI] [PubMed] [Google Scholar]
- 13.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 14.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi KH, Park YJ, Koong SS, Kim JH, Na DG, Ryu JS, et al. Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Korean J Otorhinolaryngol-Head Neck Surg. 2011;54:8–36. [Google Scholar]
- 16.Reiners C, Schumm-Draeger PM, Geling M, Mastbaum C, Schönberger J, Laue-Savic A, et al. [Thyroid gland ultrasound screening (Papillon Initiative). Report of 15 incidentally detected thyroid cancers] Internist (Berl) 2003;44:412–419. doi: 10.1007/s00108-003-0884-x. [DOI] [PubMed] [Google Scholar]
- 17.Brander AE, Viikinkoski VP, Nickels JI, Kivisaari LM. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology. 2000;215:801–806. doi: 10.1148/radiology.215.3.r00jn07801. [DOI] [PubMed] [Google Scholar]
- 18.Mizukami Y, Michigishi T, Nonomura A, Yokoyama K, Noguchi M, Hashimoto T, et al. Autonomously functioning (hot) nodule of the thyroid gland. A clinical and histopathologic study of 17 cases. Am J Clin Pathol. 1994;101:29–35. doi: 10.1093/ajcp/101.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Tan GH, Gharib H, Reading CC. Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med. 1995;155:2418–2423. doi: 10.1001/archinte.155.22.2418. [DOI] [PubMed] [Google Scholar]
- 20.Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696–700. doi: 10.7326/0003-4819-133-9-200011070-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, et al. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J Ultrasound Med. 2009;28:1685–1692. doi: 10.7863/jum.2009.28.12.1685. [DOI] [PubMed] [Google Scholar]
- 22.Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN. Thyroid follicular carcinoma: sonographic features of 50 cases. AJR Am J Roentgenol. 2010;194:44–54. doi: 10.2214/AJR.09.3195. [DOI] [PubMed] [Google Scholar]
- 23.Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J Radiol. 2007;8:192–197. doi: 10.3348/kjr.2007.8.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusić Z, Becker DV, Saenger EL, Paras P, Gartside P, Wessler T, et al. Comparison of technetium-99m and iodine-123 imaging of thyroid nodules: correlation with pathologic findings. J Nucl Med. 1990;31:393–399. [PubMed] [Google Scholar]
- 25.Reschini E, Catania A, Ferrari C, Bergonzi M, Paracchi A, Raineri P. Comparison of pertechnetate and radioiodine thyroid scintiscans in thyroid disease. J Nucl Biol Med. 1993;37:12–17. [PubMed] [Google Scholar]
- 26.Belfiore A, Sava L, Runello F, Tomaselli L, Vigneri R. Solitary autonomously functioning thyroid nodules and iodine deficiency. J Clin Endocrinol Metab. 1983;56:283–287. doi: 10.1210/jcem-56-2-283. [DOI] [PubMed] [Google Scholar]