Abstract
AIM: To explore the association of neurotensin receptor 1 (NTSR1) with inflammatory bowel diseases (IBD) and colitis-associated neoplasia.
METHODS: NTSR1 was detected by immunohistochemistry in clinical samples of colonic mucosa with IBD colitis, colitis-associated raised low-grade dysplasia (LGD) including dysplasia-associated lesions or masses (DALMs, n = 18) and adenoma-like dysplastic polyps (ALDPs, n = 4), colitis-associated high-grade dysplasia (HGD, n = 11) and colitis-associated colorectal carcinoma (CACRC, n = 13), sporadic colorectal adenomatous polyp (SAP, n = 17), and sporadic colorectal carcinoma (SCRC, n = 12). The immunoreactivity of NTSR1 was semiquantitated (as negative, 1+, 2+, and 3+) and compared among different conditions.
RESULTS: NTSR1 was not detected in normal mucosa but was expressed similarly in both active and inactive colitis. LGD showed a significantly stronger expression as compared with non-dysplastic colitic mucosa, with significantly more cases showing > 2+ intensity (68.75% in LGD vs 32.26% in nondysplastic mucosa, P = 0.001). However, no significant difference existed between DALMs and ALDPs. CACRC and HGD showed a further stronger expression, with significantly more cases showing 3+ intensity than that in LGD (61.54% vs 12.50% for CACRC vs LGD, P = 0.022; 58.33% vs 12.50% for CACRC/HGD vs LGD, P = 0.015). No significant difference existed between colitis-associated and non-colitic sporadic neoplasia.
CONCLUSION: NTSR1 in colonic epithelial cells is overexpressed in IBD, in a stepwise fashion with sequential progress from inflammation to dysplasia and carcinoma.
Keywords: Neurotensin, Neurotensin receptor, Inflammatory bowel diseases, Dysplasia, Colitis-associated neoplasia, Dysplasia-associated lesion or mass, Sporadic adenoma, Colorectal carcinoma
Core tip: Neurotensin receptor 1 (NTSR1) in colonic epithelial cells is overexpressed in inflammatory bowel diseases, in a stepwise fashion with the sequential progress from inflammation to low-grade dysplasia, high-grade dysplasia, and carcinoma. Both colitis-associated and sporadic dysplasia/carcinoma showed a similar pattern of NTSR1 overexpression. NTSR1 could be a potential pharmacological target in the treatment of inflammatory bowel diseases and prevention of colitis-associated neoplasia.
INTRODUCTION
Neurotensin (NTS) is a 13-amino-acid peptide secreted by neurons and specialized endocrine cells (N-cells) in the small intestine, which acts as a paracrine and endocrine modulator of various gut functions. The biological activities of NTS are mediated mainly through the high-affinity neurotensin receptor 1 (NTSR1), a member of the G-protein-coupled receptor family[1].
Many studies have suggested a possible role of NTS/NTSR-1 in the pathogenesis of inflammatory bowel disease (IBD) and colitis-associated neoplasia. The NTS/NTSR-1 signaling pathway has a complex dual effect (both proinflammatory and proregenerative) in the regulation of intestinal mucosal inflammation. NTS/NTSR-1 enhances the progression of acute colonic inflammation. NTS enhances mast cell degranulation and neutrophil recruitment[2-4]. NTSR-1 expression is increased in the human colonic mucosa with active ulcerative colitis (UC)[5,6] as well as in rodent colitis induced by Clostridium difficile toxin A[4] or by dextran sulfate sodium (DSS)[6], whereas pretreatment of NTSR-1 antagonist SR48692 inhibits the inflammatory changes[4]. Both NTS and NTSR1 expression are also increased in the mesenteric fat of mice during trinitrobenzenesulfonic-acid-induced colitis[7]. Moreover, NTS/NTSR1 activation in colonocytes stimulates interleukin (IL)-8 secretion from colonocytes through activating GTPase-mediated nuclear factor κ-light-chain-enhancer of activated B cells, mitogen-activated protein kinase, and protein kinase C[8-11]. NTS/NTSR-1 augments mucosal healing and regeneration following chronic colitis[5,6]. Pretreatment with NTSR1 antagonist worsens the severity of experimentally induced colitis and delays mucosal healing, whereas coadministration of exogenous NTS exerts the opposite effect[5,6,12,13]. NTS also stimulates colonic epithelial cell migration and proliferation through COX-2 gene expression[5,6].
As a part of their diverse bioactivities, NTS/NTSR1 signaling is also involved in the early carcinogenesis and progression of colonic carcinoma. First, NTSR-1 is overexpressed in colonic neoplasms. A previous investigation of one of the present authors (Gui X) showed a stepwise increase in NTSR-1 mRNA with progression of colonic adenoma to adenocarcinoma[14]. Second, NTS is an epidermal growth factor (EGF)-like factor in a number of tumors[15-18]; it transactivates EGF receptor, hence it works synergistically with EGF[19,20]. Third, NTSR-1 gene activation is linked with the Wnt/APC/Tcf/β-catenin pathway in colonic neoplasia. Upregulation of NTSR-1 in colorectal adenocarcinoma is the result of Wnt/APC pathway activation, and the increased NTSR-1 expression correlates with β-catenin cytosolic or nuclear accumulation. Additionally, NTSR-1 gene can be activated by agents that cause β-catenin cytosolic accumulation[21]. Recently, it was also found that NTSR1 activation stimulates the expression of miRNAs 21 and 155 in colonocytes, in the experimentally induced colonic cancer (HCT-116 xenograft tumors) in mice as well as in human colonic carcinoma tissues[22].
It is well known that long-standing colitis (IBD) predisposes to the development of colorectal carcinoma (CRC). The relative risk of the development of CRC in IBD patients is 10-40-fold higher compared to that in the general population[23]. This so-called colitis-associated colorectal cancer (CACRC) is the most serious complication and the major cause of death of IBD patients. The carcinogenesis of CACRC is believed to be initiated and/or promoted by persistent active inflammation of colorectal mucosa[24,25] in the inflammation-dysplasia-carcinoma sequence, which differs from the adenoma-carcinoma sequence in sporadic CRC.
The bidirectional effect of NTS/NTSR1 on colonic mucosal inflammation-particularly the stimulation of cytokines/chemokines production and promotion of epithelial cell growth-makes NTS/NTSR1 signaling a possible unique link between chronic mucosal inflammation and carcinogenesis. For example, IL-8 [or chemokine CXC ligand (CXCL)8], an inflammatory component as a chemotactic factor for leukocytes, affects cancer (including colon cancer) progression through mitogenic, angiogenic, and motogenic effects[26]. The secretion of IL-8 can be stimulated by NTSR1 activation in colonic epithelial cells under either inflammatory or neoplastic conditions[27].
Taken together, it seems reasonable to postulate that NTSR1 in colonic epithelial cells is upregulated in IBD and that NTSR1 overexpression may play a role in the development of colitis-associated dysplasia/neoplasia. In this study, we analyzed the expression of NTSR1 in human colonic mucosa with various pathological changes characteristic of IBD and IBD-associated dysplasia and carcinoma. In order to demonstrate whether the change in NTSR1 was specific to IBD-associated neoplasia, we compared it to the sporadic colorectal neoplasia that developed in non-colitic patients.
MATERIALS AND METHODS
Study subjects
All cases were retrieved retrospectively from the surgical pathology files of the Calgary Laboratory Services in 2009 and 2010. The study was approved by the Research Committee of Calgary Laboratory Services, and ethical approval was granted by the University of Calgary Conjoint Health Research Ethics Board. The classification and grading of IBD, dysplasia and carcinoma were carried out on hematoxylin and eosin (HE)-stained slides, based on the morphological features and according to the standardized histological consensus criteria used widely in clinical pathology practice. An attempt was made to distinguish, based on the strict morphological criterion currently available, between dysplasia-associated lesions or masses (DALMs) and adenoma-like dysplastic polyps (ALDPs) for the raised dysplastic lesions developed in a background of IBD. DALMs were defined as lesions that met all of the following criteria: (1) location within areas of chronic colitis; (2) irregularly elevated and broad-based with indistinct boundaries; and (3) surrounding mucosa also dysplastic, and in a full-thickness or “bottom-up” pattern of dysplasia. ALDPs were defined by the presence of well-circumscribed typical adenoma-looking polyps seen in the colitic regions; mostly in a “top-down” pattern of dysplasia. ALDPs are considered most likely to be sporadic adenoma.
Four separate groups of cases were included in the study.
Group 1: Eighteen colectomy cases of long-standing IBD complicated by colorectal neoplasia (14 males, 4 females, aged 26-84 years), including 13 UC, three Crohn’s disease, and two indeterminate colitis. In this group, we looked for sequential histological changes of inflammation/dysplasia/carcinoma. In each case, the tissue blocks were selected from those with proven histology of normal/unremarkable colonic mucosa (n = 16), active colitis (n = 16), inactive colitis (n = 14), raised low-grade dysplasia (LGD, n = 16), high-grade dysplasia (HGD, n = 11), and adenocarcinoma (CACRC, n = 13). For the LGD lesions, 12 DALMs and four ALDPs were subgrouped.
Group 2: Eighteen colonoscopic biopsies of DALMs detected in longstanding IBD patients (10 males, 8 females, aged 23-68 years) for neoplasia surveillance.
Group 3: Seventeen randomly selected biopsies of sporadic colorectal adenomatous polyps detected in non-IBD patients (10 males, 7 females, aged 35-79 years) for colon cancer screening.
Group 4: Twelve randomly selected cases of colectomy for sporadic colorectal adenocarcinoma (SCRC) detected in non-IBD patients (7 males, 5 females, aged 42-85 years).
Detection of NTR-1 in colonic epithelial cells
The expression of NTSR1 was detected by immunohistochemistry of deparaffinized sections using the avidin-biotin-peroxidase complex method. The formalin-fixed paraffin-embedded tissue sections were pretreated in CINTec Epitope Retrieval Solution (10 mmol/L Tris/1 mmol/L EDTA, pH 9.0) for 20 min at 95-100 °C, and then cooled down slowly to room temperature. The NTSR1 antibody was a rabbit polyclonal antibody (Imgenex, San Diego, CA, United States) against the third cytoplasmic domain of human NTSR1. All slides were stained with Ventana Nexes IHC autostainer at 1:40 dilution using UltraView Universal DAB Detection (Ventana 760-500). Immunoreactivity of NTSR1 appeared in a cytoplasmic pattern. Only the surface and cryptal epithelial cells of colonic mucosa were analyzed (in order to eliminate the variability of mononuclear cells in lamina propria). The positivity and intensity of the immunoreactivity of NTSR1 were semiquantitated independently by two pathologists as absent (negative), weak (1+), moderate (2+), and strong (3+). If the signal intensity was heterogeneous, the level assigned was based on the intensity in at least 50% of tumor cells or epithelial cells. A comparison of NTSR1 expression was analyzed between the different groups (active vs inactive colitis, dysplastic vs non-dysplastic lesions, DALMs vs ALDPs, colitis-associated dysplasia vs sporadic non-colitic dysplasia, and colitis-associated CRC vs sporadic non-colitic CRC).
Statistical analysis
Analysis of variance test was used to determine the statistical significance of the NTSR1 expression intensity between different groups. Differences were considered significant if P was < 0.05.
RESULTS
NTSR1 expression in colonic mucosa is associated with stepwise progression from colitis to LGD, HGD and carcinoma
Within the 18 colectomy specimens, relatively normal (uninvolved/colitis-spared) mucosa was identified in 12 cases. NTSR1 was not detected in any of the samples of normal mucosa. In the colitic mucosa, however, NTSR1 was detected in 30 out of 31 representative tissue samples and expressed similarly in both active and inactive colitis, as shown in Table 1 and Figure 1.
Table 1.
Neurotensin receptor 1 expression in 18 cases of inflammatory bowel diseases with colectomy n (%)
| Histology |
Case |
P value | |||
| Negative | 1+ | 2+ | 3+ | ||
| Normal | 12 (100.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Colitis | 1 (3.23) | 20 (64.52) | 10 (32.36) | 0 (0.00) | |
| Active | 0 (0.00) | 12 (75.00) | 4 (25.00) | 0 (0.00) | |
| Inactive | 1 (6.67) | 8 (53.33) | 6 (40.00) | 0 (0.00) | 0.3381 |
| LGD | 0 (0.00) | 3 (18.75) | 11 (68.75) | 2 (12.50) | 0.0072 |
| DALM | 0 (0.00) | 3 (25.00) | 7 (58.33) | 2 (16.67) | |
| ALDP | 0 (0.00) | 0 (0.00) | 4 (100.00) | 0 (0.00) | 0.2981 |
| HGD | 0 (0.00) | 1 (9.09) | 4 (36.36) | 6 (54.55) | 0.0633 |
| CACRC | 0 (0.00) | 1 (7.69) | 4 (30.77) | 8 (61.54) | 0.0223 |
Within two subgroups (active vs inactive);
As compared with colitis;
As compared with low-grade dysplasia (LGD). HGD: High-grade dysplasia; DALM: Dysplasia-associated lesions or masses; ALDP: Adenoma-like dysplastic polyps; CACRC: Colitis-associated colorectal carcinoma.
Figure 1.
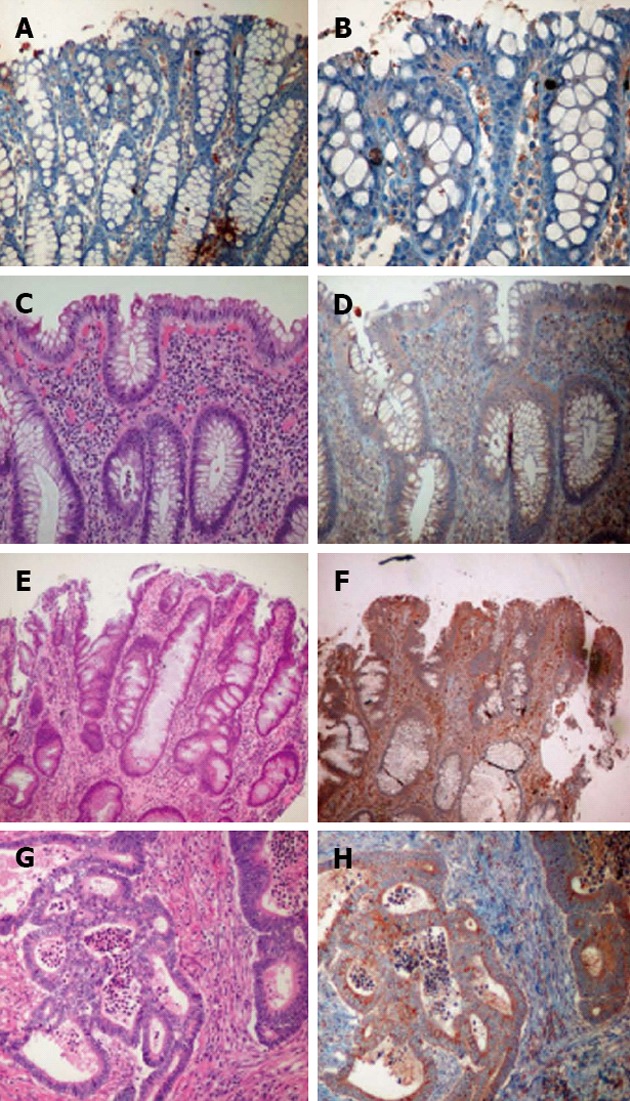
Neurotensin receptor 1 expression in colonic mucosa under different conditions. A and B: Normal colonic mucosa; C and D: Active chronic colitis; E and F: Dysplasia-associated lesions or masses with low-grade dysplasia; G and H: Invasive adenocarcinoma (hematoxylin and eosin histology and neurotensin receptor 1 expression immunohistochemistry).
LGD lesions were identified in 16 of the 18 cases. The epithelium with LGD showed a significantly stronger expression of NTSR1 as compared to the non-dysplastic colitic mucosa, with most cases showing a ≥ 2+ intensity (68.75% in LGD vs 32.26% in non-dysplastic mucosa, P = 0.001) but fewer cases showing a 1+ intensity (18.75% in LGD vs 64.52% in non-dysplastic mucosa, P = 0.007). However, no significant difference existed between DALMs and ALDPs, as shown in Table 1 and Figures 1 and 2.
Figure 2.
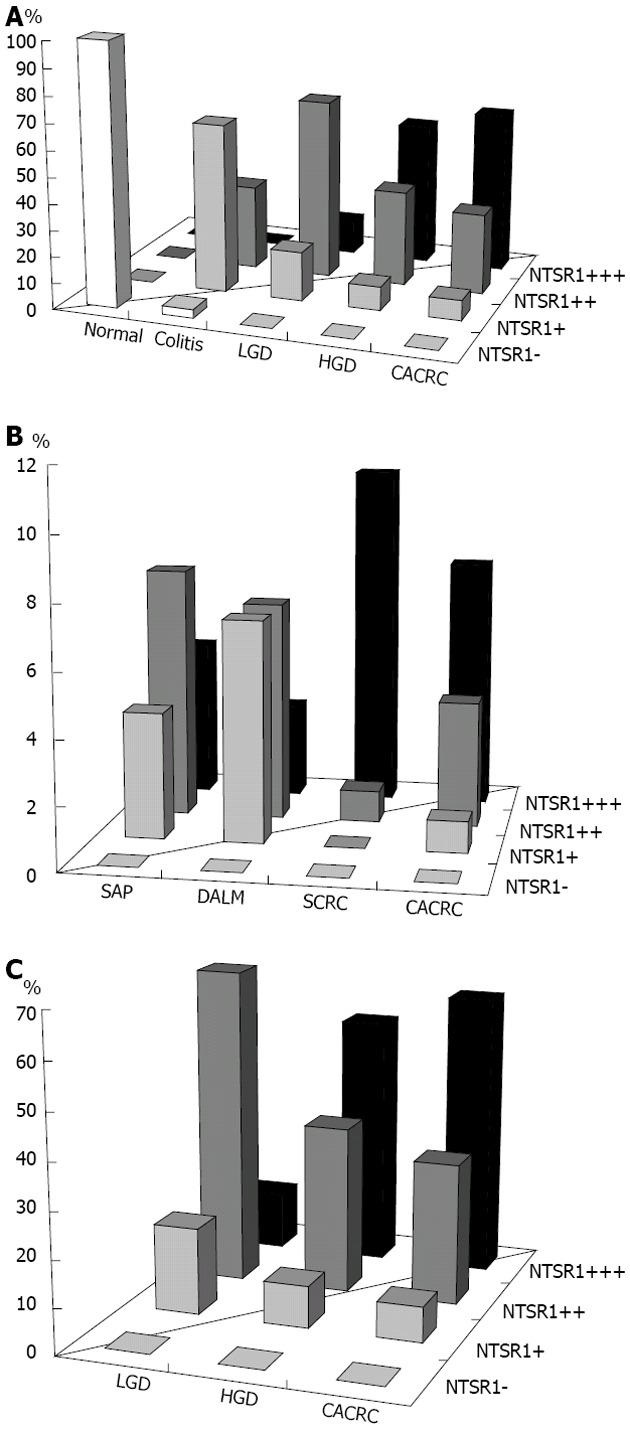
Neurotensin receptor 1 expression. A: Neurotensin receptor 1 (NTSR1) expression in colonic mucosa under different conditions in cases of colectomy for inflammatory bowel diseases (IBD) (percentage of cases in each subgroup); B: NTSR1 expression in colonic mucosa with dysplasia and carcinoma in cases of colectomy for IBD (percentage of cases in each subgroup); C: Comparison of colonic mucosal NTSR1 expression in sporadic neoplasia and colitis-associated neoplasia (percentage of cases in each subgroup). LGD: Low-grade dysplasia; HGD: High-grade dysplasia; DALM: Dysplasia-associated lesions or masses; CACRC: Colitis-associated colorectal carcinoma; SAP: Sporadic colorectal carcinoma; SCRC: Sporadic colorectal adenomatous polyp.
HGD was identified in 11 cases, and CACRC was identified in 13. As shown in Table 1 and Figures 1 and 2, expression of NTSR1 in the CACRC and HGD samples was stronger, with significantly more cases showing a 3+ intensity than in LGD of both DALMs and ALDPs (61.54% vs 12.50% for CACRC vs LGD, P = 0.022; 58.33% vs 12.50% for CACRC/HGD vs LGD, P = 0.015). However, no significant difference existed between CACRC and HGD (P = 0.942).
NTSR1 expressed similarly between DALMs and non-colitic sporadic adenoma
In the cases of colitis-associated DALMs (n = 18) and non-colitis sporadic adenomas (n = 17), the increased expression of NTSR1 showed a similar pattern, as shown in Table 2 and Figure 2.
Table 2.
Neurotensin receptor 1 expression n (%)
| Negative | 1+ | 2+ | 3+ | |
| SAP | 0 | 4 (23.53) | 8 (47.06) | 5 (29.41) |
| DALM | 0 | 7 (41.42) | 7 (41.42) | 3 (17.65) |
| SCRC | 0 | 0 | 1 (8.33) | 11 (91.67) |
| CACRC | 0 | 1 (7.69) | 4 (30.77) | 8 (61.54) |
Comparison between colitis-associated dysplasia/carcinoma and sporadic dysplasia/carcinoma. Sporadic colorectal adenomatous polyp (SCRC) vs colitis-associated colorectal carcinoma (CACRC), P = 0.198; Dysplasia-associated lesions or masses (DALMs) vs CACRC, P = 0.028; DALMs vs sporadic colorectal carcinomas (SAPs), P = 0.50.
NTSR1 expressed similarly between colitis-associated and sporadic CRC
The increased expression of NTSR1 also showed a similar pattern to that in CACRC and in conventional sporadic colorectal carcinoma (SCRC, n = 12), as shown in Table 2 and Figure 2.
DISCUSSION
Through the detection of NTSR1 expression directly in human colonic mucosa with various IBD-related pathologies and those with sporadic colonic neoplasia in non-IBD patients, the present study demonstrated that both active and inactive IBD colitis upregulated NTSR1 in colonic epithelial cells; colitis-associated LGD to HGD and carcinoma was associated with stepwise higher expression of NTSR1; and the overexpression of NTSR1 showed a similar pattern in colitis-associated and non-colitic sporadic dysplasia/neoplasia, which suggests that NTSR1 is commonly unregulated in colonic neoplasia with or without a background of colitis.
The first two findings support the hypothesis that the upregulation of NTSR1 is involved in IBD inflammation and colitis-associated neoplasia. The findings corroborate various studies carried out in the past in animal models and ex vivo systems. In a similar study reported by Bossard et al[28], identical findings were shown with a slightly different methodology. Their study also demonstrated that coexpression of NTS/NTSR1 is present in a majority of the inflammatory and neoplastic/dysplastic lesions, suggestive of a self-activation of NTSR1 secondary to increased production of ligands; and β-catenin nuclear translocation is seen in a minority of the dysplastic and carcinomatous lesions in which no NTS was detected, suggestive of a different pathway.
To the best of our knowledge, the finding that NTSR1 is similarly overexpressed in colitis-associated dysplasia/neoplasia and sporadic dysplasia/neoplasia in non-IBD patients has not been reported previously. This finding indicates that NTSR1 is a neoplastic marker irrespective of its underlying etiology. In other words, the NT/NTSR1 signaling pathway is intrinsic and common to the tumorigenesis of all colorectal carcinomas, regardless of the tumor-promoting factors or the predisposing/initiation processes. This finding and interpretation are supported by an animal study reported recently by Bugni et al[29]. They developed a chemical-carcinogen-induced colonic adenoma by administration of azoxymethane to mice with or without DSS-induced colitis. NTSR1-deficient (gene knockout) mice had a < 50% chance, compared to wild-type mice, of developing colonic adenoma in the absence of colitis (i.e., a model of sporadic colonic neoplasia). The difference, however, disappeared in the mice that had colitis (, a model of colitis-associated colonic neoplasia), even though significantly higher levels of IL-6 and CXCL2 (the mouse homolog of IL-8, both known as tumor-promoting cytokines) were seen in the latter. Our study, as well as that of Bugni et al[29], suggests that NTS/NTSR1 are not particularly responsible for the link between chronic inflammation and neoplasia in IBD, although it is commonly involved in the entire multistep process as an intrinsic regulator. The tumorigenetic process in colitis-associated dysplasia/neoplasia appears far more complex. However, it is still possible that increased NTSR1 expression associated with pre-existent or coexistent chronic colitis may further enhance the carcinogenesis.
It was noted in a minority of cases that NTSR1 expression was less upregulated, which occurred nonspecifically for each of the conditions and in different cases. We have no solid explanation for these relative negative cases. It is possible that NTSR1 expression in the epithelium is regulated by multiple factors, including a variety of cytokines and other gut peptides in the local mucosa or circulation that are not always the same in different patients.
Overall, our findings further provide a rationale for exploring the anti-NTSR1 approach in the treatment of IBD and in the chemoprevention of IBD-associated colorectal neoplasia as well as the treatment of sporadic colonic neoplasia. Hopefully, the development of clinically useful NTSR1 blockers will become a reality in the near future with the recent better understanding of the chemical structure of NTSR1[30].
ACKNOWLEDGMENTS
Ms. Michelle Darago provided very helpful technical support.
COMMENTS
Background
Inflammatory bowel diseases (IBD) are chronic and debilitating inflammatory conditions of the colon, which also increase the relative risk of colorectal cancer. Identification of the factors associated with both mucosal inflammation and colitis-associated carcinogenesis could lead to novel treatments.
Research frontiers
Neurotensin (NTS) is a 13-amino-acid peptide that acts as a paracrine and endocrine modulator of various gut functions. Its bioactivities are mediated mainly through the high-affinity neurotensin receptor 1 (NTSR1). The NTS/NTSR1 signaling pathway has dual effects (both proinflammatory and proregenerative) in the regulation of intestinal mucosal inflammation. NTS/NTSR1 signaling is also involved in the carcinogenesis and progression of colonic carcinoma. It is suggested that NTSR1 in colonic epithelial cells is upregulated in IBD and NTSR1 overexpression may play a role in the development of colitis-associated dysplasia/neoplasia.
Innovations and breakthroughs
The present study used colonic tissue samples to detect the expression of NTSR1 in the context of various pathological conditions of IBD, with a focus on NTSR1 expression in the progressive changes from active inflammation to low-grade dysplasia, high-grade dysplasia, and carcinoma. A stepwise increase in NTSR1 expression was identified in the sequential progression. Moreover, a similar pattern of NTSR1 overexpression in colitis-associated and sporadic dysplasia/neoplasia was also determined for the first time.
Applications
The findings provide a rationale for exploring the anti-NTSR1 approach in the treatment of IBD and in the chemoprevention of IBD-associated colorectal neoplasia, a clinically useable anti-NTSR1 agent becomes available in the near future.
Terminology
Colitis-associated dysplasia and carcinoma develop in association with the longstanding chronic colitis in IBD patients. It is now well recognized that this type of colorectal carcinogenesis is clearly initiated and/or promoted by chronic, persistent and repetitively active mucosal inflammation. This type of colorectal carcinoma (CRC) develops and progresses in an inflammation-dysplasia-carcinoma sequence and therefore differs from the adenoma-carcinoma sequence in the sporadic CRC.
Peer review
The study investigates the relationship between NTSR1 expression in IBD and the possibility of its association with mucosal inflammation and colitis-associated neoplasia. They found a strong correlation with the progression from normal mucosa to colitis, degree of dysplasia, and carcinoma. The methodological approach was correct and the findings are interesting.
Footnotes
Supported by Calgary Laboratory Services Internally Supported Research, RS09-533
P- Reviewer Iera E S- Editor Gou SX L- Editor Kerr C E- Editor Zhang DN
References
- 1.Najimi M, Maloteaux JM, Hermans E. Cytoskeleton-related trafficking of the EAAC1 glutamate transporter after activation of the G(q/11)-coupled neurotensin receptor NTS1. FEBS Lett. 2002;523:224–228. doi: 10.1016/s0014-5793(02)02981-2. [DOI] [PubMed] [Google Scholar]
- 2.Cooke HJ. Neuroimmune signaling in regulation of intestinal ion transport. Am J Physiol. 1994;266:G167–G178. doi: 10.1152/ajpgi.1994.266.2.G167. [DOI] [PubMed] [Google Scholar]
- 3.Garrido JJ, Arahuetes RM, Hernanz A, De la Fuente M. Modulation by neurotensin and neuromedin N of adherence and chemotaxis capacity of murine lymphocytes. Regul Pept. 1992;41:27–37. doi: 10.1016/0167-0115(92)90511-r. [DOI] [PubMed] [Google Scholar]
- 4.Castagliuolo I, Wang CC, Valenick L, Pasha A, Nikulasson S, Carraway RE, Pothoulakis C. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkotou E, Espinoza DO, Torres D, Karagiannides I, Kosteletos S, Savidge T, O’Brien M, Pothoulakis C. Melanin-concentrating hormone (MCH) modulates C difficile toxin A-mediated enteritis in mice. Gut. 2009;58:34–40. doi: 10.1136/gut.2008.155341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brun P, Mastrotto C, Beggiao E, Stefani A, Barzon L, Sturniolo GC, Palù G, Castagliuolo I. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–G629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 7.Koon HW, Kim YS, Xu H, Kumar A, Zhao D, Karagiannides I, Dobner PR, Pothoulakis C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc Natl Acad Sci USA. 2009;106:8766–8771. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokkotou E, Moss AC, Torres D, Karagiannides I, Cheifetz A, Liu S, O’Brien M, Maratos-Flier E, Pothoulakis C. Melanin-concentrating hormone as a mediator of intestinal inflammation. Proc Natl Acad Sci USA. 2008;105:10613–10618. doi: 10.1073/pnas.0804536105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates interleukin-8 expression through modulation of I kappa B alpha phosphorylation and p65 transcriptional activity: involvement of protein kinase C alpha. Mol Pharmacol. 2005;67:2025–2031. doi: 10.1124/mol.104.010801. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D, Kuhnt-Moore S, Zeng H, Wu JS, Moyer MP, Pothoulakis C. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-kappa B pathways. Am J Physiol Cell Physiol. 2003;284:C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]
- 11.Law IK, Murphy JE, Bakirtzi K, Bunnett NW, Pothoulakis C. Neurotensin-induced proinflammatory signaling in human colonocytes is regulated by β-arrestins and endothelin-converting enzyme-1-dependent endocytosis and resensitization of neurotensin receptor 1. J Biol Chem. 2012;287:15066–15075. doi: 10.1074/jbc.M111.327262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akcan A, Muhtaroglu S, Akgun H, Akyildiz H, Kucuk C, Sozuer E, Yurci A, Yilmaz N. Ameliorative effects of bombesin and neurotensin on trinitrobenzene sulphonic acid-induced colitis, oxidative damage and apoptosis in rats. World J Gastroenterol. 2008;14:1222–1230. doi: 10.3748/wjg.14.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil. 2009;21:439–450. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui X, Guzman G, Dobner PR, Kadkol SS. Increased neurotensin receptor-1 expression during progression of colonic adenocarcinoma. Peptides. 2008;29:1609–1615. doi: 10.1016/j.peptides.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Ishizuka J, Townsend CM, Thompson JC. Neurotensin regulates growth of human pancreatic cancer. Ann Surg. 1993;217:439–445; discussion 446. doi: 10.1097/00000658-199305010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seethalakshmi L, Mitra SP, Dobner PR, Menon M, Carraway RE. Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate. 1997;31:183–192. doi: 10.1002/(sici)1097-0045(19970515)31:3<183::aid-pros7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Sethi T, Langdon S, Smyth J, Rozengurt E. Growth of small cell lung cancer cells: stimulation by multiple neuropeptides and inhibition by broad spectrum antagonists in vitro and in vivo. Cancer Res. 1992;52:2737s–2742s. [PubMed] [Google Scholar]
- 18.Evers BM. Neurotensin and growth of normal and neoplastic tissues. Peptides. 2006;27:2424–2433. doi: 10.1016/j.peptides.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Zhao D, Zhan Y, Koon HW, Zeng H, Keates S, Moyer MP, Pothoulakis C. Metalloproteinase-dependent transforming growth factor-alpha release mediates neurotensin-stimulated MAP kinase activation in human colonic epithelial cells. J Biol Chem. 2004;279:43547–43554. doi: 10.1074/jbc.M401453200. [DOI] [PubMed] [Google Scholar]
- 20.Hassan S, Carraway RE. Involvement of arachidonic acid metabolism and EGF receptor in neurotensin-induced prostate cancer PC3 cell growth. Regul Pept. 2006;133:105–114. doi: 10.1016/j.regpep.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Souazé F, Viardot-Foucault V, Roullet N, Toy-Miou-Leong M, Gompel A, Bruyneel E, Comperat E, Faux MC, Mareel M, Rostène W, et al. Neurotensin receptor 1 gene activation by the Tcf/beta-catenin pathway is an early event in human colonic adenomas. Carcinogenesis. 2006;27:708–716. doi: 10.1093/carcin/bgi269. [DOI] [PubMed] [Google Scholar]
- 22.Bakirtzi K, Hatziapostolou M, Karagiannides I, Polytarchou C, Jaeger S, Iliopoulos D, Pothoulakis C. Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141:1749–1761.e1. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 24.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 25.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006;12:5346–5355. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossard C, Souazé F, Jarry A, Bezieau S, Mosnier JF, Forgez P, Laboisse CL. Over-expression of neurotensin high-affinity receptor 1 (NTS1) in relation with its ligand neurotensin (NT) and nuclear beta-catenin in inflammatory bowel disease-related oncogenesis. Peptides. 2007;28:2030–2035. doi: 10.1016/j.peptides.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Bugni JM, Rabadi LA, Jubbal K, Karagiannides I, Lawson G, Pothoulakis C. The neurotensin receptor-1 promotes tumor development in a sporadic but not an inflammation-associated mouse model of colon cancer. Int J Cancer. 2012;130:1798–1805. doi: 10.1002/ijc.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, et al. Structure of the agonist-bound neurotensin receptor. Nature. 2012;490:508–513. doi: 10.1038/nature11558. [DOI] [PMC free article] [PubMed] [Google Scholar]


