Abstract
Cyclic nucleotide-gated (CNG) channels are critical for sensory transduction in retinal photoreceptors and olfactory receptor cells; their activity is modulated by phosphoinositides (PIPn) such as phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3). An achromatopsia-associated mutation in cone photoreceptor CNGA3, L633P, is located in a carboxyl (COOH)-terminal leucine zipper domain shown previously to be important for channel assembly and PIPn regulation. We determined the functional consequences of this mutation using electrophysiological recordings of patches excised from cells expressing wild-type and mutant CNG channel subunits. CNGA3-L633P subunits formed functional channels with or without CNGB3, producing an increase in apparent cGMP affinity. Surprisingly, L633P dramatically potentiated PIPn inhibition of apparent cGMP affinity for these channels. The impact of L633P on PIPn sensitivity depended on an intact amino (NH2) terminal PIPn regulation module. These observations led us to hypothesize that L633P enhances PIPn inhibition by altering the coupling between NH2- and COOH-terminal regions of CNGA3. A recombinant COOH-terminal fragment partially restored normal PIPn sensitivity to channels with COOH-terminal truncation, but L633P prevented this effect. Furthermore, coimmunoprecipitation of channel fragments, and thermodynamic linkage analysis, also provided evidence for NH2-COOH interactions. Finally, tandem dimers of CNGA3 subunits that specify the arrangement of subunits containing L633P and other mutations indicated that the putative interdomain interaction occurs between channel subunits (intersubunit) rather than exclusively within the same subunit (intrasubunit). Collectively, these studies support a model in which intersubunit interactions control the sensitivity of cone CNG channels to regulation by phosphoinositides. Aberrant channel regulation may contribute to disease progression in patients with the L633P mutation.
Keywords: CNG, channel, CLZ, phosphoinositide, PIP3, PIP2, cGMP, cAMP, achromatopsia
cyclic nucleotide-gated (CNG) channels of retinal cone photoreceptors are crucial to color and high-acuity vision (5, 29). CNG channels are nonselective cation channels, the opening of which is controlled by intracellular cGMP in photoreceptors (10, 28). Phototransduction is initiated by light activation of opsins, which leads to lower cGMP levels and closure of the CNG channels. Thus, light signals are translated into electrical responses through CNG channels, resulting in a hyperpolarization of retinal photoreceptors. Each protein subunit of CNG channels consists of six transmembrane domains (S1–S6), a P-loop between S5 and S6, and cytoplasmic amino (NH2)- and carboxyl (COOH)-terminal regions. The COOH-terminal region of each subunit is composed of a COOH-linker region, a cyclic nucleotide-binding domain (CNBD), and the post-CNBD region. Binding of cGMP to the CNBD induces a conformational change that is allosterically coupled to channel opening via gating rearrangements of the C-linker, the inner helix (S6), the pore helix, and the channel selectivity filter (7, 12, 60). Native cone CNG channels are tetrameric proteins formed by CNGA3 and CNGB3 subunits (2, 14, 65). The stoichiometry of cone CNG channel subunits is thought to be 2:2 (42, but also see 8), in contrast to the 3:1 ratio of CNGA1 to CNGB1 subunits in rod CNG channels (51, 69, 66). One structural determinant contributing to assembly of CNG channels is the carboxyl-terminal leucine zipper (CLZ) domain within the post-CNBD region. The CLZ domains are α-helices composed of heptad repeats of periodic hydrophobic residues. Previous studies have shown that the CLZ domain can assemble via coiled-coil interactions and thus may help control CNG channel subunit arrangement (51, 68, 69).
CNG channels do not desensitize to continuous cyclic nucleotide exposure, but they are regulated by various mechanisms, including via Ca2+-calmodulin (CaM) in olfactory and visual sensory cells (16, 25, 47, 57). This feedback regulation is thought to contribute, for example, to the ability of cone photoreceptors to adapt to a wide range of background light intensities (9). For cone CNG channels, the Ca2+-CaM mediated decrease in apparent ligand affinity is only modest (23, 40) and cannot account for the considerable Ca2+-dependent inhibition of native cone channels (21). A recent study suggests that another Ca2+-binding protein, CNG-modulin, may represent the authentic Ca2+-dependent modulator of cone CNG channels in striped bass (46). However, other mechanisms for regulation of CNG channels have been revealed and might interact with the aforementioned Ca2+-dependent modulators to adjust cone CNG channel activity.
Phosphoinositides (PIPn), a family of acidic phospholipids within cell membranes, have been demonstrated to be modulators of many types of ion channels, including CNG channels (13, 53). Receptor-mediated changes in the concentration of PIPn species, particularly phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-bisphosphate (PIP3), underlie many forms of ion channel regulation. For most channels, these phosphoinositides serve to support or promote channel activity. For photoreceptor and olfactory CNG channels, however, phosphoinositides primarily inhibit channel activity (3, 64). In photoreceptors, PIPn levels are controlled by light, intracellular Ca2+, paracrine signals, and circadian oscillators (6, 19, 22, 31). Thus, PIPn inhibition of CNG channels has the potential to explain several key aspects of photoreceptor physiology. We have found previously that cone CNGA3 + CNGB3 channels are inhibited by PIP2 and PIP3, showing a rightward shift in the cGMP dose-response relationship (a decrease in the apparent cGMP affinity) after PIPn application (4, 7a). In addition, we have recently defined two structural components that are essential for PIPn regulation of cone CNG channels; these are located within the cytoplasmic NH2- and the COOH-terminal regions of CNGA3, respectively (7a).
Mutations in the genes encoding both CNGA3 and CNGB3 subunits of cone photoreceptor CNG channels have been linked to debilitating eye diseases in humans, including complete and incomplete achromatopsia, cone dystrophy, and inherited macular degeneration (1, 39, 45, 52, 54, 63). These pathologies are associated with cone dysfunction and progressive photoreceptor cell death, producing loss of central, high-acuity vision and day blindness (52). Human CNG channelopathies highlight the essential role of these proteins for photoreceptor health. Electrophysiological and biochemical characterization of CNGA3 and CNGB3 mutations has revealed both loss- and gain-of function defects (1, 32, 41, 48), suggesting that tight control of CNG channel activity is critical for the normal function of cone photoreceptors. While the basic functional effects for many disease-associated mutations have been defined, the potential impact of disease-associated mutations on cone CNG channel regulation has remained unknown.
Here, we report the functional consequences of a previously uncharacterized achromatopsia-associated mutation: L633P in human CNGA3 (17). This leucine residue is located in the part of the protein after the CNBD, within the COOH-terminal CLZ domain described above. The fundamental activation properties of cone CNG channels were only modestly altered by L633P. However, L633P conspicuously enhanced the PIP2 or PIP3 inhibition of cGMP-dependent channel gating, an effect that required the PIPn regulation module located within the NH2-terminal region of CNGA3. On the basis of in vitro coimmunoprecipitation studies and thermodynamic linkage analysis, we propose that L633P alters the interaction between NH2- and COOH-terminal regions of CNGA3 and therefore potentiates the NH2-terminal component of PIPn regulation within CNGA3 subunits.1
MATERIALS AND METHODS
Molecular biology.
Human CNGB3 was cloned as previously described (40). Human CNGA3 was a gift of K.-W. Yau (Johns Hopkins University, Baltimore, MD). For heterologous expression in Xenopus oocytes, CNGA3 and CNGB3 coding sequences were subcloned into pGEMHE, where they are flanked by the Xenopus β-globin gene 5′- and 3′-untranslated regions. The procedures for making point mutations and generating amino-terminal fusions of enhanced green fluorescent protein (eGFP) with CNGA3 were described previously (40). All mutations were confirmed by DNA sequencing. For expression of channel fragments as GST-tagged proteins, CNGA3 sequences were amplified and inserted into pGEX-5X2; coding sequences for these GST fusion proteins were subsequently subcloned into pGEMHE. For concatenated cDNA constructs, the stop codon for the leading subunit and the start codon for the trailing subunit were replaced by a short linker sequence (IAGGGGGRARLPA), combining the coding sequences for the two subunits into a single open reading frame (42). For expression in Xenopus oocytes, cRNA was synthesized in vitro from linearized channel-subunit cDNAs using an upstream T-7 promoter and the mMessage mMachine kit (Ambion, Austin, TX).
Patch-clamp electrophysiology.
Xenopus laevis oocytes were isolated as previously described (42) and injected with ∼20 ng of cRNA. The animal use protocols were consistent with the recommendations of the American Veterinary Medical Association and were approved by the Institutional Animal Care and Use Committee (IACUC) of Washington State University. Patch-clamp experiments were performed using an Axopatch 200B amplifier (Axon Instruments, Union City, CA) in the inside-out configuration. Initial pipette resistances were 0.4–0.8 MΩ. Intracellular and extracellular solutions contained 130 mM NaCl, 0.2 mM EDTA, and 3 mM HEPES (pH 7.2). Cyclic nucleotides were added to intracellular solution as needed. Macroscopic patch current density was calculated by estimating the patch area A (μm2) from the initial pipette resistance R (MΩ) based on the equation A = 12.6(1/R + 0.018) (50). Intracellular solutions were changed using an RSC-160 rapid solution changer (Molecular Kinetics, Indianapolis, IN). Solutions containing PIP3 or PIP2 analogs, diC8-PI (3,4,5) P3 or diC8-PI (4,5) P2 (Echelon Biosciences, Salt Lake City, UT), were prepared with FVPP (a phosphatase inhibitor cocktail) as previously described (4). Recordings were made at 20 to 22°C.
Protein biochemistry.
After 4 days of incubation at 16°C, oocytes that were injected with cRNA were homogenized in oocyte lysis buffer (20 mM Tris·HCl, 2 mM NaPO4, 150 mM NaCl, 1 mM EDTA, 0.5% vol/vol Triton X-100, 0.5% vol/vol NP-40, 0.5% vol/vol CHAPS, pH 7.4). The homogenization buffer was supplemented with 1 protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN) per 10 ml buffer. We used 20–40 oocytes per sample group and 20 μl lysis buffer per oocyte. The homogenate was incubated on ice for 1 h and then centrifuged two to three times at 20,000 g for 12 min at 4°C to separate the supernatant from insoluble material and the floating fat layer. Soluble lysates were precleared using 10 μl of a 50% slurry of control agarose beads (Thermo Scientific, Waltham, MA). After 1 h of incubation with rocking, the samples with control beads were centrifuged at 2,000 g for 3 min and the supernatants were removed. The supernatants again were centrifuged at 20,000 g for 10 min and the supernatants were collected again. Then we applied 15 μl 50% slurry of anti-GFP beads (Vector Laboratories, Burlingame, CA) to the supernatant. After 2 h of incubation with rocking, the anti-GFP beads were gently pelleted and washed four times with lysis buffer. Protein complexes that interacted with anti-GFP beads were eluted using 2 × NuPAGE sample buffer (Invitrogen, Carlsbad, CA). Protein samples and the control oocyte lysates were then separated under reducing conditions using SDS-PAGE in 4–12% Bis-Tris gels. After gel electrophoresis, proteins were transferred to a nitrocellulose filter by electroblotting with the NuPAGE transfer buffer system (Invitrogen). The transferred proteins were detected via Western blotting using a mouse anti-GST monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at a dilution of 1:1,000 in TTBS solution composed of 1% milk, 20 mM Tris·HCl (pH 7.5), 500 mM NaCl, and 0.05% Tween 20. The blotted proteins from control oocyte lysates also were probed using a 1:2,500 diluted mouse anti-GFP antibody (Clontech, Palo Alto, CA) and the same anti-GST antibody. Anti-actin antibodies (Chemicon International, Temecula, CA) were used to ensure that the same amounts of input proteins were used. Chemiluminescent detection with horseradish peroxidase-conjugated anti-mouse IgG secondary antibodies was performed using the Super-Signal West Dura Extended Duration detection kit (Pierce).
Data analysis.
Data were acquired and analyzed using Pulse (HEKA Elektronik, Lambrecht, Germany), Igor (Wavemetrics, Portland, OR), and SigmaPlot/SigmaStat (Systat Software, San Jose, CA). Currents in the absence of cyclic nucleotide were subtracted. For channel activation by cyclic nucleotides, dose-response data were fitted with the Hill equation: I/Imax = [cNMP]h/(K1/2h + [cNMP]h), where I is the current amplitude, Imax is the maximum current elicited by saturating concentration of ligand, [cNMP] is the ligand concentration, K1/2 is the concentration of cNMP producing half-maximal current, and h is the Hill coefficient. To confirm the formation of heteromeric CNG channels, block by 25 μM l-cis-diltiazem (Sigma-Aldrich, St. Louis, MO) in 1 mM cGMP at +80 mV was measured. The Gibbs free energy of the overall reaction of CNG channel opening, ΔG, was calculated using the equation ΔG = −RT ln (1/K1/2h) (7, 30), where R is the gas constant, 1.987 cal·K−1·mol−1, and T is the temperature in degrees Kelvin. We calculated the phosphoinositide-induced change in free energy difference for the channel opening reaction, ΔΔG, using the following: ΔΔG = ΔGPIPn − ΔGcont = RT ln {[K1/2,PIPnh(PIPn)]/[K1/2,conth(cont)]}, where cont (control) and PIPn indicate the values before and after PIP2 or PIP3 application. The interaction energy ΔΔΔintG for the thermodynamic linkage analysis was calculated as follows: ΔΔΔintG = (ΔΔGPA-LP,L633P − ΔΔGPA-LP) − (ΔΔGL633P − ΔΔGWT), where ∣ΔΔΔintG∣ = 0 indicates no interaction and purely additive effects. In our study, ΔΔΔintG > 1 kcal/mol was considered significantly interacted or coupled (30, 62). Data parameters are expressed as means ± SE of n experiments unless otherwise indicated. Statistical significance was determined by using Student's t-test or Mann-Whitney U-test.
RESULTS
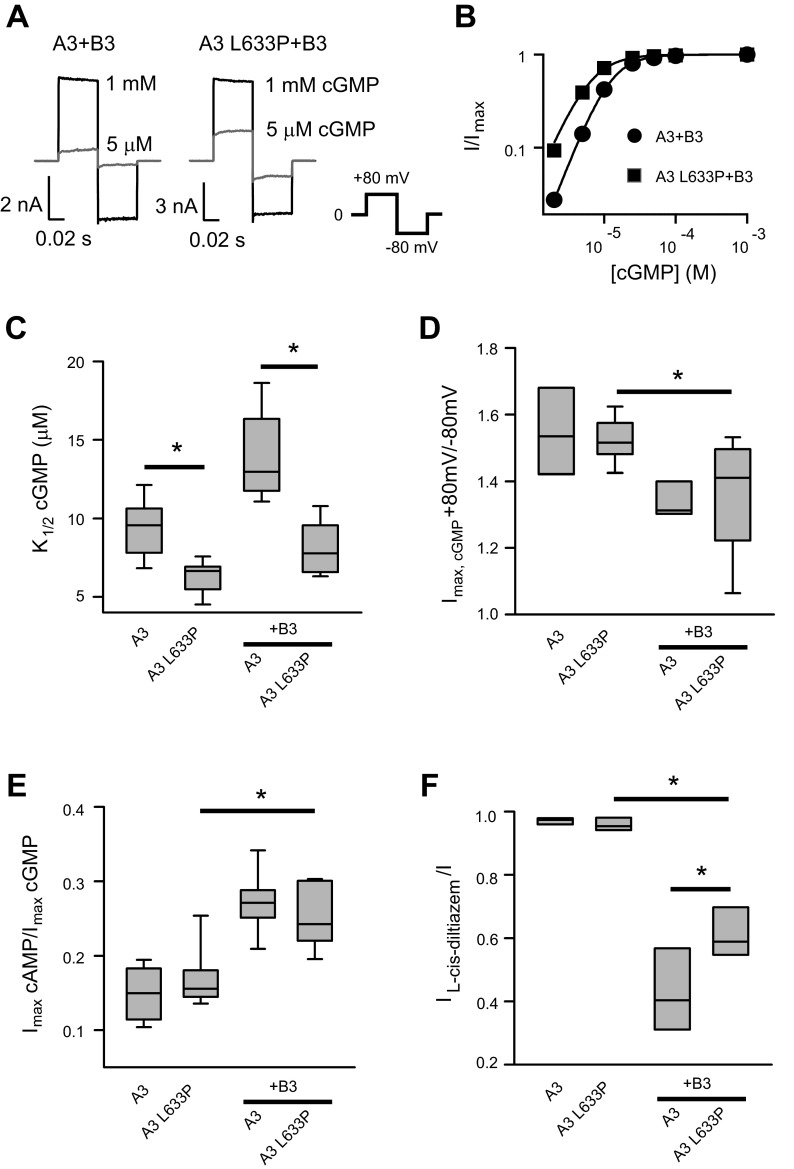
We examined the functional effects of the disease-associated mutation L633P, which is located in the carboxyl-terminal leucine zipper (CLZ) domain of CNGA3 subunits, on the basic properties of channels formed by cone photoreceptor CNGA3 (A3) and CNGB3 (B3) subunits. A3 subunits can assemble to form functional homomeric (A3-only) channels; B3 subunits alone cannot form functional channels. However, the expression of A3 plus B3 subunits together generates heteromeric channels that essentially recapitulate the properties of native channels found in cone photoreceptor outer segments. Previous studies (68) have shown that CLZ-defective CNGA3 mutants have a decreased capacity to form heteromeric channels with CNGB1, suggesting that the CLZ domain is important for selective heteromeric assembly of CNG channels. L633P introduces a structurally distinct and conformationally rigid amino acid proline, which is commonly found as disruptors of protein secondary-structural components (such as α-helices) (34, 38). Therefore, we hypothesized that L633P could exert a profound disruption of the CLZ domain, potentially altering channel gating and regulation. Our approach to studying this mutation was to express homomeric and heteromeric wild-type or L633P-containing channels in Xenopus oocytes, followed by examining the properties of these channels in excised, inside-out membrane patches using electrophysiological recordings and application of the channel activators cGMP or cAMP to the cytoplasmic face of the patch. We found that the average maximal current densities (current/patch area) produced by 1 mM cGMP (Fig. 1A and Table 1) were significantly decreased by L633P: for heteromeric channels, current densities were 146.6 ± 49.6 pA/μm2 for A3 + B3 and 30.0 ± 7.2 pA/μm2 for A3-L633P + B3 (P < 0.05); for homomeric channels, current densities were 587.0 ± 244.5 pA/μm2 for wild-type A3 and 111.2 ± 33.3 pA/μm2 for A3-L633P (P < 0.05). Decreased current density for L633P-containing channels indicates that the mutation impaired but did not prevent functional expression of CNG channels.
Fig. 1.
Summary of effects of L633P mutation on basic properties of homomeric and heteromeric cone cyclic nucleotide-gated (CNG) channels. A: representative currents elicited by saturating 1 mM cGMP (black) and 5 μM cGMP (gray) for heteromeric A3 + B3 channels composed of A3 wild-type or A3-L633P. Currents were elicited by voltage steps from a holding potential of 0 mV to + 80 mV, then to −80 mV and 0 mV. Leak currents in the absence of cyclic nucleotide were subtracted. B: representative cGMP dose-response relationships for activation of heteromeric A3 + B3 and A3 L633P + B3 channels, fitted with the Hill equation. C: K1/2,cGMP values for homomeric A3 channels and heteromeric A3 + B3 channels with or without the L633P mutation are shown using a box plot. The horizontal lines within the boxes indicate the median values; the bottom and top of the boxes show the 25th and 75th percentiles of the data, respectively; the ends of whiskers show the 5th and 95th percentiles of the data. D and E: outward current rectification (Imax,cGMP + 80 mV/−80 mV; D) and relative cAMP efficacy (Imax,cAMP/Imax,cGMP; E) of A3 and A3 + B3 channels were not significantly altered by L633P. F: l-cis-diltiazem inhibition of maximal cGMP current was attenuated by L633P. The ratio of cGMP current in the presence and absence of 25 μM l-cis-diltiazem (IL-cis-diltiazem/I) was 0.42 ± 0.05 (n = 8) for wild-type A3 + B3 channels and 0.62 ± 0.03 (n = 8) for A3-L633P + B3 channels. *P < 0.05.
Table 1.
Effects of PIPn application on gating parameters of CNGA3 or CNGA3 + CNGB3 channels
| Control |
PIP2 |
PIP3 |
|||||
|---|---|---|---|---|---|---|---|
| Imax, A | K1/2, cGMP, μM | h | K1/2, cGMP, μM | h | K1/2, cGMP, μM | h | |
| A3 | 1.4 ± 0.5 × 10−8 (9) | 8.6 ± 0.5 (9) | 1.9 ± 0.0 | 9.1 ± 0.5 (4) | 1.8 ± 0.1 | 7.7 ± 0.7 (5) | 2.0 ± 0.0 |
| A3 L633P | 3.0 ± 1.0 × 10−9 (13) | 5.9 ± 0.3 (13) | 1.9 ± 0.0 | 12.5 ± 0.9 (8)* | 1.7 ± 0.0 | 13.4 ± 0.7 (5)* | 1.9 ± 0.1 |
| A3+B3 | 3.9 ± 1.7 × 10−9 (9) | 13.5 ± 0.9 (9) | 2.0 ± 0.0 | 23.2 ± 3.0 (5)* | 1.6 ± 0.1 | 18.8 ± 2.0 (4)* | 1.8 ± 0.2 |
| A3 L633P+B3 | 2.0 ± 0.6 × 10−9 (12) | 8.1 ± 0.4 (12) | 1.9 ± 0.0 | 20.6 ± 1.8 (7)* | 1.6 ± 0.1 | 19.9 ± 3.1 (5)* | 1.6 ± 0.1 |
| A3 613X | 1.9 ± 0.7 × 10−10 (10) | 13.2 ± 0.1 (10) | 1.6 ± 0.1 | 44.4 ± 5.1 (4)* | 1.3 ± 0.1 | 47.1 ± 3.2 (6)* | 1.4 ± 0.1 |
| A3 624X | 7.3 ± 1.7 × 10−10 (8) | 6.8 ± 0.4 (8) | 2.0 ± 0.0 | 15.6 ± 3.9 (3)* | 1.5 ± 0.0 | 14.9 ± 0.5 (5)* | 1.5 ± 0.0 |
| A3 635X | 2.8 ± 1.0 × 10−9 (4) | 7.1 ± 0.5 (4) | 1.8 ± 0.0 | ND | ND | 6.6 ± 0.2 (4) | 1.7 ± 0.1 |
| A3 635X, L633P | 1.4 ± 0.5 × 10−9 (5) | 6.7 ± 0.2 (5) | 2.0 ± 0.0 | ND | ND | 11.7 ± 0.7 (5) | 1.6 ± 0.1 |
| A3 E627R | 1.1 ± 0.6 × 10−9 (9) | 8.8 ± 0.5 (9) | 2.0 ± 0.0 | 8.9 ± 1.1 (4) | 2.0 ± 0.0 | 10.1 ± 1.2 (5) | 1.8 ± 0.1 |
| A3 E627,628R | 1.9 ± 0.5 × 10−10 (9) | 11.3 ± 0.7 (9) | 1.8 ± 0.0 | 17.0 ± 2.4 (5)* | 1.5 ± 0.1 | 15.7 ± 1.4 (4)* | 1.9 ± 0.0 |
| A3 E631R | 2.2 ± 0.8 × 10−9 (4) | 7.7 ± 0.4 (4) | 1.9 ± 0.1 | ND | ND | 7.9 ± 0.4 (4) | 1.9 ± 0.1 |
| A3 N7R-A | 2.7 ± 0.4 × 10−11 (4) | 8.9 ± 1.2 (4) | 1.6 ± 0.2 | ND | ND | 8.7 ± 0.8 (4) | 1.8 ± 0.1 |
| A3 N7R-A,L633P | 3.5 ± 0.5 × 10−11 (11) | 6.3 ± 0.3 (11) | 1.8 ± 0.1 | 6.6 ± 0.4 (5) | 1.5 ± 0.1 | 6.9 ± 0.3 (6) | 1.9 ± 0.1 |
| A3 N7RA,L633P+B3 | 1.1 ± 0.3 × 10−10 (8) | 9.5 ± 0.4 (8) | 1.6 ± 0.0 | 9.3 ± 1.2 (4) | 1.6 ± 0.0 | 9.5 ± 1.7 (4) | 1.6 ± 0.1 |
| A3 PA-LP | 1.5 ± 0.6 × 10−9 (10) | 6.1 ± 0.3 (10) | 1.8 ± 0.1 | 11.0 ± 0.4 (6)* | 1.6 ± 0.0 | 9.8 ± 1.4 (4)* | 1.8 ± 0.1 |
| A3 PA-LP, L633P | 5.0 ± 1.3 × 10−10 (8) | 4.5 ± 0.3 (8) | 1.8 ± 0.0 | 10.6 ± 0.9 (8)* | 1.7 ± 0.0 | ND | ND |
| A3//A3 | 6.8 ± 3.4 × 10−9 (4) | 8.2 ± 0.5 (4) | 2.0 ± 0.0 | 8.7 ± 0.9 (4) | 2.0 ± 0.0 | ND | ND |
| A3//A3 L633P | 2.4 ± 0.5 × 10−9 (4) | 5.3 ± 0.3 (4) | 1.6 ± 0.0 | 12.3 ± 1.3 (4)* | 1.6 ± 0.0 | ND | ND |
| A3 N7R-A//A3 L633P | 5.8 ± 1.0 × 10−11 (4) | 7.9 ± 0.4 (4) | 1.7 ± 0.0 | 8.1 ± 0.4 (4) | 1.7 ± 0.0 | ND | ND |
| A3//A3 N7R-A,L633P | 4.8 ± 0.5 × 10−11 (4) | 6.3 ± 0.3 (4) | 1.8 ± 0.1 | 12.6 ± 0.8 (4)* | 1.7 ± 0.0 | ND | ND |
Values are means ± SE. The numbers in parentheses after the values indicate number of patches (n); K1/2, cGMP was determined using the Hill equation. h, Hill coefficient; Imax, maximum current; ND, not determined; CNG, cyclic nucleotide-gated; K1/2, cGMP, concentration of nucleoside 3′,5′-cyclic monophosphate producing half-maximal current; PIP2, phosphatidylinositol (4,5)-biphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate.
Significant difference between Hill parameters before and after phosphoinositide (PIPn) treatment (P < 0.05).
Next we determined the effect of L633P on channel activation properties. Native photoreceptor CNG channels operate under low cGMP concentrations in the dark, and these levels fall in response to light stimulation of the cells (43). Therefore, alterations in CNG channel ligand sensitivity can have profound consequences for channel activity under physiological conditions. We found that L633P significantly increased the current activated by a subsaturating concentration of cGMP (5 μM) relative to the current activated by a saturating concentration of cGMP (1 mM) (Fig. 1A); this low concentration of cGMP approximates the intracellular cGMP levels within vertebrate photoreceptors in the dark. The L633P mutation increased the apparent cGMP affinity (decreased K1/2,cGMP) for heteromeric CNGA3 + CNGB3 channels (Fig. 1, B and C). Similarly, L633P increased the apparent cGMP affinity for homomeric CNGA3 channels (Fig. 1C).
As L633P may influence channel assembly, we sought to confirm that the mutation did not prevent the formation of heteromeric channels. To accomplish this, we investigated three features that are used to functionally distinguish between heteromeric versus homomeric CNG channels. First, we tested the outward rectification of currents mediated by L633P-containing channels. Heteromeric A3 + B3 channels characteristically produce less outwardly rectifying currents (outward current at +80 mV versus inward current at −80 mV) in saturating concentrations of cGMP compared with homomeric A3-only channels (42). L633P had no significant effect on current rectification for either homomeric (Fig. 1D) or heteromeric channels (Fig. 1, A and D), suggesting that CNGA3 subunits containing the mutation were able to assemble into heteromeric channels with CNGB3 subunits. Second, we examined the ratio of current elicited by a saturating concentration of cAMP relative to that produced by saturating cGMP (Imax,cAMP/Imax,cGMP) for L633P-containing channels. For cone photoreceptor CNG channels, cAMP is a partial agonist; heteromeric A3 + B3 channels are characterized by a greater Imax,cAMP/Imax,cGMP ratio compared with homomeric A3-only channels (42). The Imax,cAMP/Imax,cGMP ratio was not significantly altered by L633P (Fig. 1E), again indicating that A3-L633P was able to form functional heteromeric channels. Finally, we examined l-cis-diltiazem inhibition of cGMP-induced currents. l-cis-diltiazem block represents another reporter for the formation of functional heteromeric cone CNG channels, as channel block is minimal in the absence of CNGB3 (IL-cis-diltiazem/I = 0.94 ± 0.02 for homomeric A3 channels) (42). Heteromeric A3-L633P + B3 channels showed decreased l-cis-diltiazem inhibition in 1 mM cGMP compared with wild-type heteromeric channels (Fig. 1F). However, diltiazem inhibition for A3-L633P + B3 channels was significantly greater than homomeric (wild-type or L633P) channels (P = 0.001). Together, these results show that L633P altered the gating properties of both homomeric and heteromeric channels, producing increased sensitivity to cGMP. In addition, the results suggest a slight impairment of heteromeric channel formation, presumably due to disruption of the CLZ domain.
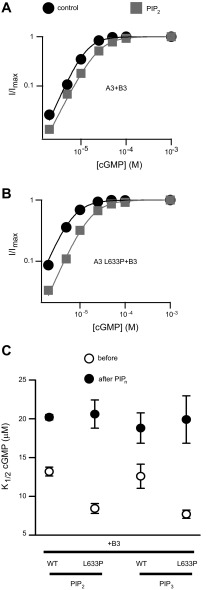
Since the L633P mutation is located in a region of CNGA3 that we have previously shown to be important for regulation by phosphoinositides, we predicted that L633P might alter the PIPn sensitivity of cone CNG channels. To test this prediction, we applied 1 μM diC8-PI (3,4,5) P3 (PIP3) or 10 μM diC8-PI (4,5) P2 (PIP2) to A3-L633P + B3 channels. The rational for using these concentrations of PIPn analogs was based on the following. First, generic membrane levels of free PIP2 in cells are thought to be ∼10 μM, and PIP3 levels are expected to be at least tenfold lower (15, 24, 59). Second, we have shown previously that 10 μM PIP2 and 1 μM PIP3 induce near maximal changes in K1/2,cGMP for activation of wild-type A3 + B3 channels; PIPn concentration-response relationships suggest that the approximate apparent affinities of A3 + B3 channels for natural PIP2, diC8-PIP2, and diC8-PIP3 are 2.8, 3.4, and 0.6 μM, respectively (7a). For regulation of wild-type A3 + B3 channels, 1 μM PIP3 or 10 μM PIP2 produced similar shifts in cGMP sensitivity, causing a 47.2 ± 4.2% (n = 4) or 60.7 ± 12.1% (n = 4) increase in K1/2,cGMP for channel activation, respectively (Fig. 2A). For A3-L633P + B3 channels, we found that PIP3 or PIP2 generated a much greater increase in K1/2,cGMP: 153.3 ± 22.2% (n = 5) after PIP3 and 136.8 ± 15.6% (n = 5) after PIP2 (Fig. 2B and Table 1). Next, we examined whether L633P alters the PIPn sensitivity of homomeric A3-only channels. Wild-type homomeric A3 channels are insensitive to PIP3 or PIP2 regarding apparent ligand affinity (K1/2,cGMP) but exhibit a more than twofold increase in cAMP efficacy (Imax,cAMP/Imax,cGMP ratio) after PIPn application. This PIPn-induced increase in cAMP efficacy is dependent on positively charged arginines located within the CLZ domain of CNGA3 (7a). Similar to heteromeric channels, we found that homomeric A3-L633P channels exhibited a large increase in K1/2,cGMP after PIP2 or PIP3 (Fig. 3, A and B, and Table 1). Interestingly, the PIPn-induced increase in Imax,cAMP/I,cGMP for homomeric channels was eliminated by L633P (Fig. 3, C and D). These results demonstrate that L633P dramatically alters PIPn regulation of both heteromeric and homomeric cone CNG channels.
Fig. 2.
L633P significantly enhances phosphoinositide (PIPn) sensitivity of CNGA3 + CNGB3 channels. A and B: representative cGMP dose-response relationships before (black) and after (gray) 10 μM phosphatidylinositol (4,5)-bisphosphate (PIP2) (diC8-PIP2) application for activation of heteromeric A3 + B3 channels, fitted with the Hill equation. L633P enhanced the rightward shift of K1/2,cGMP after PIP2. The Hill parameters were as follows: for A3 + B3 channels, before PIP2: K1/2,cGMP = 13.8 μM, h = 2.1; after PIP2: K1/2,cGMP = 23.9 μM, h = 1.8; for A3-L633P + B3 channels, before PIP2: K1/2 = 6.6 μM, h = 1.8; after PIP2: K1/2 = 16.4 μM, h = 1.7. C: summary illustrating the K1/2,cGMP values before and after 10 μM PIP2 (diC8-PIP2) or 1 μM PIP3 (diC8-PIP3) for heteromeric A3 + B3 or A3 L633P + B3 channels. PIP3, phosphatidylinositol 3,4,5-trisphosphate; WT, wild type.
Fig. 3.
Effects of L633P on PIPn sensitivity of homomeric CNGA3 channels. A: representative cGMP dose-response relationships before (black) and after (gray) 1 μM PIP3 application for activation of homomeric A3 channels, fitted with the Hill equation. L633P enhanced the rightward shift of K1/2 cGMP after PIP3. The Hill parameters were as follows: for A3 wild-type channels, before PIP3: K1/2,cGMP = 12.8 μM, h = 2; after PIP3: K1/2,cGMP = 10.5 μM, h = 2; for A3 L633P channels, before PIP3: K1/2,cGMP = 6.9 μM, h = 1.7; after PIP3: K1/2,cGMP = 15.4 μM, h = 1.6. B: summary of the K1/2,cGMP values before and after 10 μM PIP2 (n = 4) or 1 μM PIP3 (n = 5) for homomeric A3 and A3-L633P channels. C: representative currents elicited by saturating 1 mM cGMP (black) and 10 mM cAMP (gray), for homomeric channels composed of A3 wild-type or A3-L633P. Arrowhead indicates that application of 1 μM PIP3 increased the saturating cAMP current for wild-type channels. L633P attenuated the increase of cAMP current by PIP3. D: summary illustrating both PIP3- and PIP2-dependent changes in Imax,cAMP/cGMP for A3 and A3-L633P homomeric channels. *P < 0.05.
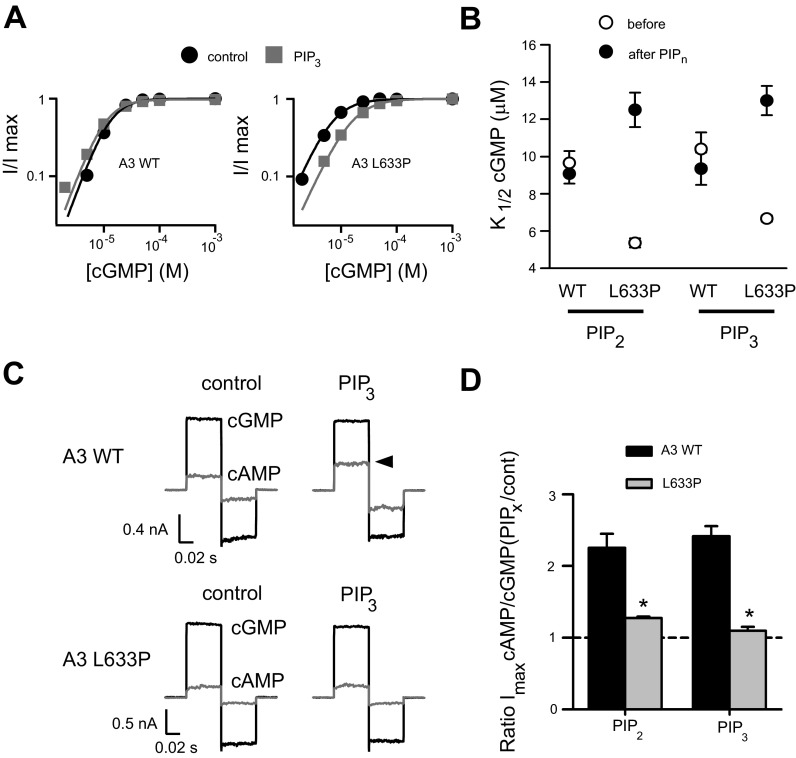
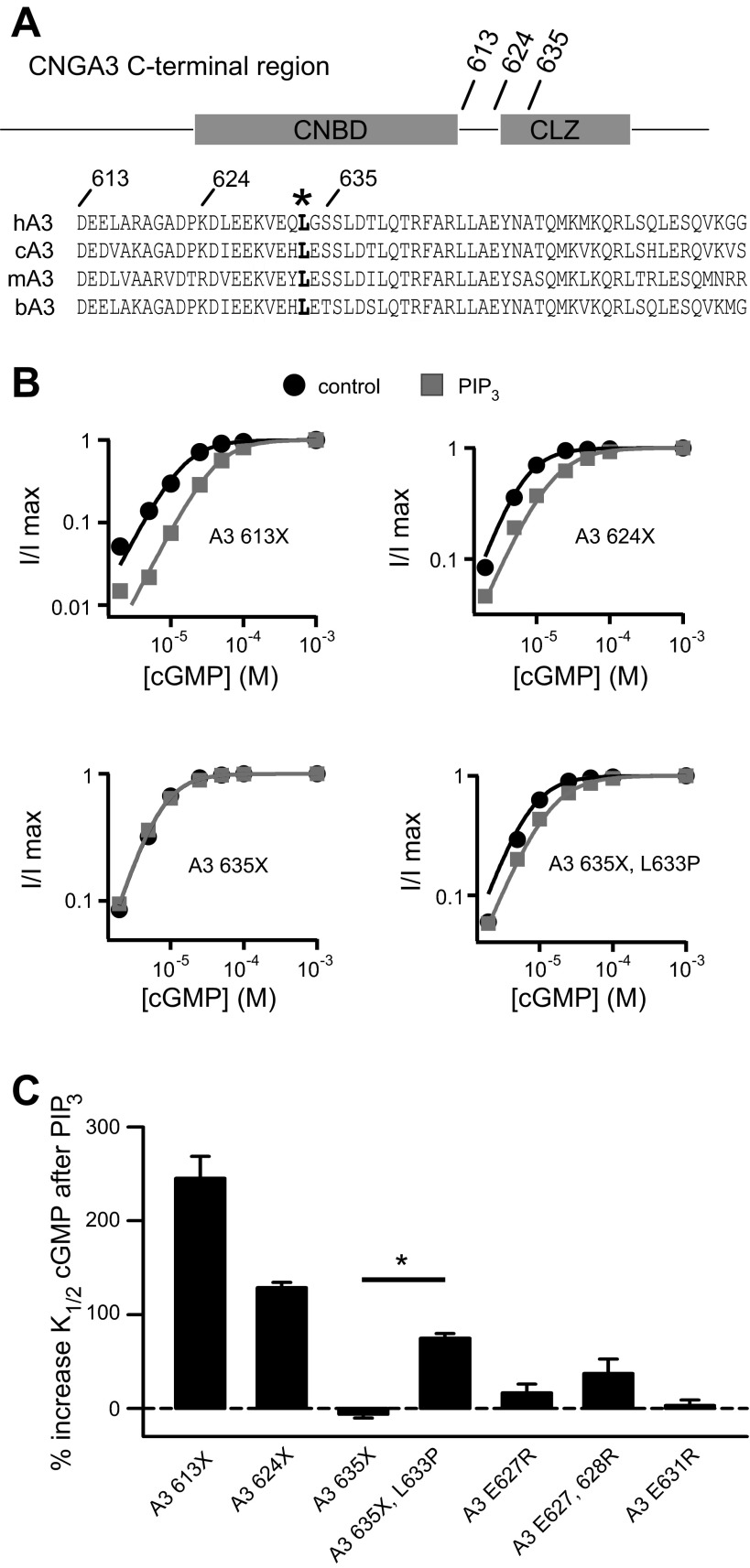
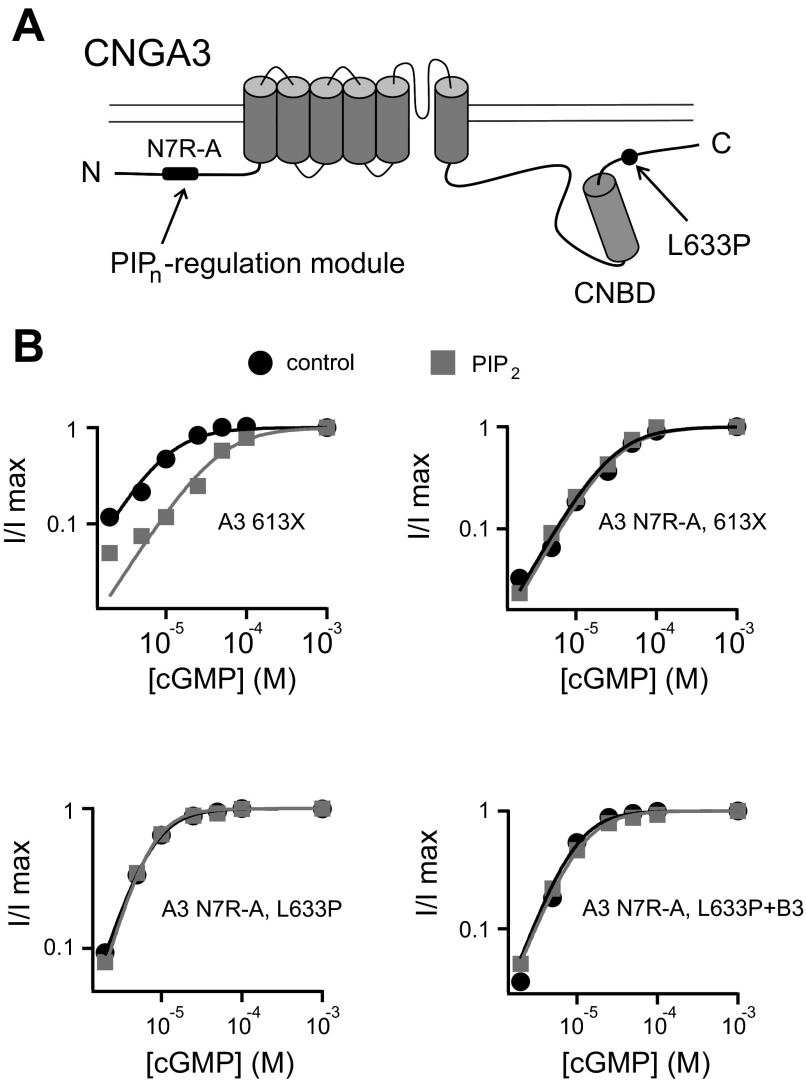
Given the location of L633P, we sought to characterize features surrounding this residue that may help control PIPn regulation of the channel. Thus, we introduced targeted deletions and mutations within the region immediately following the CNBD of CNGA3 (Fig. 4A). We have shown previously that truncations of the distal COOH-terminal region after 613 (A3–613X), but not after 635 (A3–635X), unmasked the increase in K1/2,cGMP after PIPn application for homomeric channels (Fig. 4B and Table 1). On the basis of these experiments, we hypothesized that the A3 COOH-terminal region between amino acids 613 and 635 has an attenuating effect on the NH2-terminal PIPn regulation module. Deletion of the A3 COOH-terminal region after 613 relieves this inhibition and activates the NH2-terminal regulatory module, which then is able to elicit a considerable increase in K1/2,cGMP after PIPn application. Similar to A3–613X, A3–624X exhibited sensitivity to PIP3, manifested as a decrease in apparent cGMP affinity (Fig. 4B). The difference in PIPn sensitivity for A3 624X versus 635X channels suggests that these residues bracket a structural element essential for tuning channel regulation by PIPn. In addition, we found that combining 635X with L633P generated homomeric channels that were sensitive to PIP3 (Fig. 4B), indicating that the regulatory features still present in 635X channels remain sensitive to disturbance by L633P. Furthermore, mutations of select charged residues in this region produced only a modest increase in PIPn sensitivity compared with wild-type channels (Fig. 4C): for A3 channels having single mutations E627R or E631R, the percent increase of K1/2,cGMP after 1 μM PIP3 was 16.6 ± 9.2% (n = 5) and 3.2 ± 5.7% (n = 4), respectively; for A3 channels with combined E627R and E628R mutations, the percent increase in K1/2,cGMP after PIP3 was 37.2 ± 15.3% (n = 4; P < 0.05 compared with wild-type channels). Together, these observations indicate that the COOH-terminal region between 624 and 635 may be critical for influencing PIPn sensitivity of CNGA3 channels.
Fig. 4.
CNGA3 COOH-terminal region surrounding L633P is critical for control of PIPn sensitivity. A: schematic diagram showing cyclic nucleotide-binding domain (CNBD) and COOH-terminal leucine zipper (CLZ) domain within the human CNGA3 COOH-terminal region. Sequence alignments represent human CLZ domain (hA3: 626–672) and nearby upstream sequences (hA3: 613–625) together with homologous regions of canine, mouse, and bovine CNGA3. The leucine residue L633 is highlighted in bold. B: representative cGMP dose-response curves before (black) and after (gray) PIP3 application for activation of homomeric A3 channels with COOH-terminal regions deleted, fitted with the Hill equation. C: summary illustrating the percent increase in K1/2,cGMP after PIP3 for channels with COOH-terminal truncations and mutations. *P < 0.05 for A3–635X vs. A3–635X, L633P channels.
CNGA3 subunits contain two distinct modules supporting regulation by phosphoinositides: one each in the NH2- and COOH-terminal cytoplasmic domains. On the basis of the following rationale, we hypothesized that the enhancement of PIPn sensitivity by L633P depends on the NH2-terminal PIPn regulation module. First, L633P attenuated the component of PIPn sensitivity that is mediated by the COOH-terminal regulation module, which for homomeric CNGA3-only channels is manifested as an increase in cAMP efficacy (Fig. 3, C and D). Second, L633P was able to potentiate PIPn inhibition in the context of the 635X truncation (Fig. 4C and Table 1); 635X deletes the COOH-terminal regulation module of CNGA3. To test our hypothesis, we used mutations to cripple the NH2-terminal PIPn regulation module while leaving the COOH-terminal PIPn regulation module intact. As demonstrated previously, neutralizing the positively charged arginines in this region (N7R-A: R72A, R75A, R81A, R82A, R86A, R101A, R103A) eliminated the PIPn sensitivity of A3–613X channels (Fig. 5B) and attenuated binding of an NH2-terminal domain fragment of CNGA3 to PIP3-linked agarose beads (7a). Here, we tested whether N7R-A mutations inhibited the PIPn sensitivity of A3-L633P channels similar to A3–613X channels. As expected, N7R-A abolished the PIPn-induced increase in K1/2,cGMP for both homomeric and heteromeric L633P channels (Fig. 5B); the K1/2,cGMP was not significantly altered by PIP2 application (P = 0.38 and P = 0.89, respectively, for homomeric and heteromeric channels). These results suggest that similar to COOH-terminal truncations, L633P exerts a disinhibition effect on the NH2-terminal PIPn regulation site. Silencing the NH2-terminal PIPn interaction site removes the augmentation of PIPn sensitivity caused by L633P. Furthermore, the data suggest that a potential interaction occurs between NH2- and COOH-terminal cytoplasmic regions of CNGA3 subunits.
Fig. 5.
Enhancement of PIPn sensitivity by L633P depends on the NH2-terminal PIPn regulation site of CNGA3. A: cartoon showing the PIPn regulation site within the NH2-terminal region of CNGA3; neutralization of the seven arginines within this region (N7R-A) is sufficient to prevent PIPn regulation of the channel. B: representative cGMP dose-response relationships before (black) and after (gray) 10 μM PIP2 for activation of homomeric A3–613X (Δ613–694), A3 N7R-A,613X, A3 N7R-A,L633P channels, and heteromeric A3 N7R-A, L633P + B3 channels.
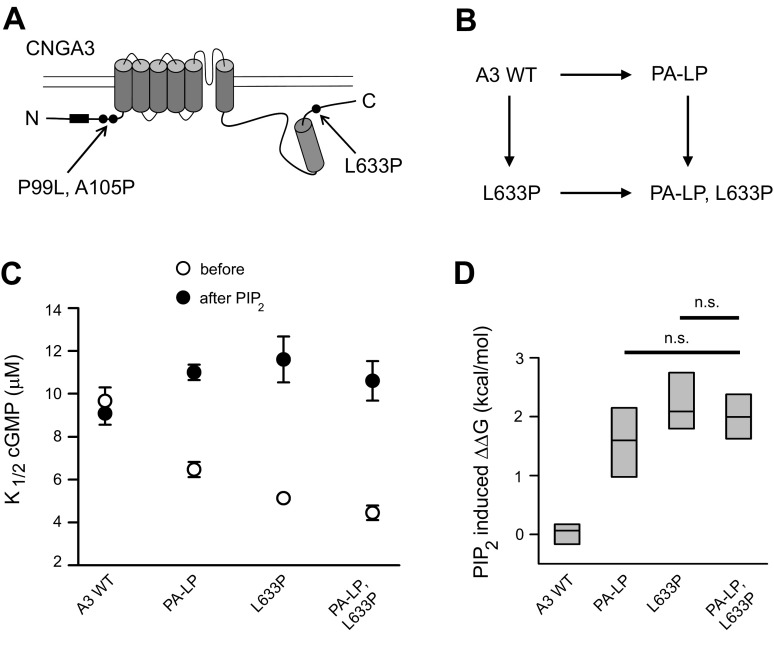
Guided by the observations described above, we hypothesized that L633P alters the coupling between NH2- and COOH-terminal regions of CNGA3 during channel activation and regulation. We used thermodynamic linkage analysis to investigate possible coupling between the NH2- and COOH-terminal domains. One previously characterized CNGA3 change, P99L, A105P (PA-LP) (18), shows an increase both in initial cGMP affinity and in PIPn sensitivity (Table 1), similar to L633P channels. In contrast to L633P, PA-LP is located within the NH2-terminal region of A3 (Fig. 6A). If interdomain NH2-COOH (N-C) coupling occurs, then the NH2-terminal PA-LP and COOH-terminal L633P mutations might alter channel gating and tune PIPn sensitivity of the channel in a convergent manner. By constituting channels with a combination of mutations, we can compare PIPn regulation of channels having single changes (PA-LP in the NH2-terminal region or L633P in the COOH-terminal region) to those with combined mutations (A3 PA-LP, L633P). The initial K1/2,cGMP for A3 PA-LP, L633P channels was significantly lower than that of A3 PA-LP, but it was not statistically different from that of A3 L633P (P = 0.204). Similarly, we found the PIP2-induced increase in K1/2,cGMP for A3 PA-LP, L633P was not statistically different from that of L633P (P = 0.751) (Fig. 6C). The changes in Gibbs free energy (ΔΔG) for channel opening reactions caused by PIPn were calculated (Fig. 6D). The ΔΔG for the combined mutations did not exhibit additive consequences when compared with the individual mutants (P > 0.05), suggesting that the effects of L633P and PA-LP on PIPn sensitivity of A3 channels overlap. Alternatively, if the effects of L633P and PA-LP on PIPn sensitivity were independent, we would have expected the PIPn-induced ΔΔG of the mutant combination to represent an approximate summation of the ΔΔG for the individual mutants. On the basis of the thermodynamic cycle shown in Fig. 6B, the interaction energy ΔΔΔintG was −1.80 ± 0.44 kcal/mol, differing substantially from zero. These results are consistent with interdomain coupling between NH2- and COOH-terminal regions of CNGA3.
Fig. 6.
Thermodynamic linkage analysis is consistent with coupling between NH2- and COOH-terminal regions of CNGA3. A: cartoon showing approximate locations of NH2-terminal P99L, A105P (PA-LP) mutations and COOH-terminal L633P mutation in CNGA3. Box represents PIPn regulation module within the NH2-terminal region. B: scheme illustrating the thermodynamic cycle. C: summary of K1/2,cGMP before and after application of 10 μM PIP2 for wild-type, individual-mutant, and combined-mutant channels. The PIP2-induced percent increase in K1/2,cGMP for A3 PA-LP, L633P was 140.7 ± 0.16% (n = 8) compared with 133.8 ± 13.4% for A3-L633P (n = 8) and 63.2 ± 5.0% for A3 PA-LP (n = 10). D: the differences (ΔΔG) are shown for the Gibbs free energy change (ΔG) of the channel opening reactions caused by PIP2 for A3 wild-type, A3 PA-LP, A3 L633P, and A3 PA-LP, L633P channels (ns, not significantly different).
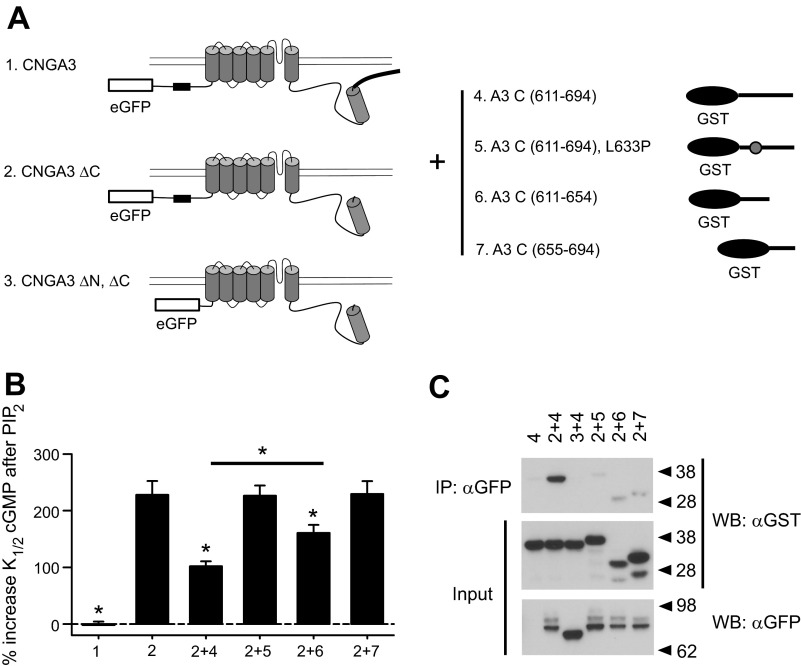
On the basis of the above results, our working hypothesis was that L633P disrupts a physical interaction between NH2- and COOH-terminal cytoplasmic domains that helps control PIPn sensitivity. To test this idea, we expressed a soluble fragment of the A3 post-CNBD region (A3C) in combination with functional A3ΔC subunits having this region truncated (613X) (Fig. 7A). We predicted that if the A3C fragment could directly interact with the truncated channel, then it would alter the response of A3ΔC channels to PIPn, attenuating the PIPn-induced increase in K1/2,cGMP and partially restoring normal PIPn sensitivity. In combination with A3ΔC, we expressed COOH-terminal fragments representing all or part of the post-CNBD region: full-length (aa 611–694), proximal (aa 611–654), and distal (aa 655–694) fragments. We found that both the full-length fragment and the proximal fragment, but not the distal fragment, significantly attenuated the increase in K1/2,cGMP for A3ΔC channels after application of PIP2 (Fig. 7B). Partial rather than full restoration of normal PIPn sensitivity may reflect low affinity and a low effective concentration for the soluble fragment compared with the situation where this domain is physically attached to the channel (high effective concentration). In contrast, coexpression of the A3C 611–694 fragment having the L633P mutation with A3ΔC channels produced PIP2 sensitivity that was not significantly different from A3ΔC channels alone (P = 0.961). Again, these data are consistent with a scenario where the proximal COOH-terminal region after the CNBD is necessary for coupling with the NH2-terminal region, and L633P can disrupt this coupling.
Fig. 7.
Coexpression of soluble CNGA3 COOH-terminal fragments supports interdomain NH2-COOH (N-C) interactions regulating PIPn sensitivity. A: cartoon showing the constructs used for coexpression experiments. CNGA3ΔC is equivalent to 613X, and CNGA3 ΔN, ΔC combines NH2-terminal Δ2–107 and COOH-terminal Δ613–694 truncations. The NH2-terminal PIPn regulation site is shown as a black box. eGFP, enhanced green fluorescent protein. B: summary showing effects of PIP2 on A3ΔC channels coexpressed with soluble A3 COOH-terminal fragments. Coexpression of A3 COOH-terminal fragments representing the region after the CNBD (aa 611–694) with A3ΔC channels significantly attenuated the increase in K1/2,cGMP after PIP2 application. However, addition of L633P to this fragment prevented this effect. The proximal A3 COOH-terminal fragment (611–654) exerted a similar effect to the full-length fragment while the more distal A3 COOH-terminal fragment (655–694) failed to attenuate the PIP2-induced increase in K1/2,cGMP for A3 ΔC channels; *P < 0.05 compared with A3ΔC channels alone. C: immunoblots are shown for coimmunoprecipitation of GST-tagged COOH-terminal fragments with eGFP-tagged A3ΔC or A3ΔNΔC channels. A3 C(611–694) was able to bind to A3ΔC (2 + 4), but not to A3ΔN,ΔC (3 + 4) in vitro. L633P prevented binding of A3 C(611–694) to A3ΔC channels (2 + 5). Both of the smaller A3 COOH-terminal fragments showed weak binding (2 + 6, 2 + 7). Approximate molecular weight markers are indicated to the right of the blots. The slower gel migration of the L633P-containing fragment (5) likely reflects a less compact structure compared with the wild-type fragment. Lower-molecular-weight bands for fragments 6 and 7 represent minor proteolytic products that retain the GST tag. Note that the top molecular weight bands shown in the bottom blot represent glycosylated CNGA3 subunits.
The putative interaction between NH2- and COOH-terminal domains of CNGA3 was further investigated using in vitro coimmunoprecipitation studies (Fig. 7C). We coexpressed enhanced GFP-tagged A3–613X subunits (eGFP-A3ΔC) with soluble GST-tagged A3 post-CNBD fragments (GST-A3C) in oocytes. Use of A3ΔC subunits is expected to prevent the homotypic interactions between intact CLZ domains previously described (69). We isolated A3ΔC channels from oocyte lysates using anti-eGFP antibodies linked to agarose beads, followed by immunoblotting the pulled-down protein complexes using anti-GST antibodies. We found that A3C (611–694) was able to bind to A3ΔC channels; the shorter COOH-terminal fragments A3C (611–654) and A3C (655–694) also exhibited (weak) interactions with A3ΔC channels in vitro. We next asked whether coimmunoprecipitation of A3C with A3ΔC requires an intact NH2-terminal domain. Thus, we coexpressed channel subunits having both NH2- and COOH-terminal regions deleted (eGFP-A3ΔN, ΔC) together with GST-A3C fragments. We found the NH2-terminal deletion eliminated the binding of the COOH-terminal fragment to the channel. Finally, we observed that the L633P mutation prevented binding of GST-A3C (611–694) fragments to eGFP-A3ΔC channels, in agreement with the electrophysiological data described above. Taken together, these observations support interactions between the NH2- and COOH-terminal regions of CNGA3 that help control PIPn sensitivity.
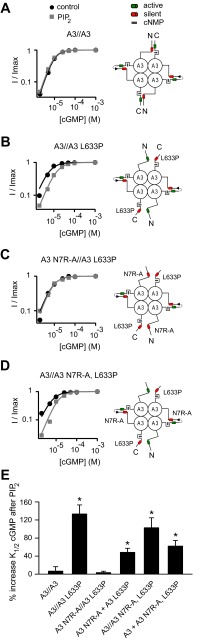
Interactions between NH2- and COOH-terminal regions of the channel may occur via contacts within the same subunit (intrasubunit) or between channel subunits (intersubunit). On the basis of our model suggesting that L633P separates or uncouples the A3 COOH-terminal region from the NH2-terminal region, resulting in unmasking of PIPn sensitivity that depends on an NH2-terminal regulation module, we designed concatenated cDNA constructs to produce tandem dimers of A3 subunits containing the L633P mutation. Tandem linkage of A3 subunits by itself did not alter PIPn regulation of the channels: the apparent cGMP affinity of A3//A3 dimers remained insensitive to 10 μM PIP2 (Fig. 8A), while A3//A3 L633P channels showed a 133.1 ± 20.2% (n = 4) increase in K1/2,cGMP (Fig. 8B). Since charge neutralizations within the NH2-terminal PIPn-regulation module (N7R-A) antagonized the PIPn sensitivity of L633P channels, we generated tandem dimers of A3 subunits with N7R-A and L633P mutations either in alternating subunits (A3 N7R-A//A3 L633P) or within the same subunit (A3//A3 N7R-A, L633P). If the N-C interactions occur within the same subunit (intrasubunit), then A3 N7R-A//A3 L633P channels are expected to be PIPn sensitive; N7R-A within one subunit would not affect the L633P-activated PIPn regulation module within the NH2-terminal region of the adjacent subunit. Conversely, if the N-C interaction occurs between subunits (intersubunit), then A3 N7R-A//A3 L633P channels are expected to be PIPn insensitive, because the NH2-terminal PIPn regulation site within one subunit that was unmasked by L633P of the adjacent subunit would be silenced by N7R-A mutations. Channels formed by A3 N7R-A//A3 L633P dimers were found to be insensitive to PIP2 regarding apparent cGMP affinity (Fig. 8C), showing only a 3.3 ± 3.2% increase in K1/2,cGMP (n = 4; P = 0.701 compared with untreated channels). We next tested A3//A3 N7R-A, L633P dimers. Since channels formed by either wild-type A3 or A3 N7R-A, L633P subunits expressed alone as homomultimers are PIPn insensitive, PIPn sensitivity observed when combining these subunits together in the same channel would provide evidence for an intersubunit interaction. Channels formed by A3//A3 N7R-A, L633P dimers exhibited a 102.6 ± 22.1% (n = 4) increase in K1/2,cGMP (Fig. 8D), indicating that L633P within one subunit was able to activate the intact PIPn-regulation module within the NH2-terminal region of an adjacent subunit, even though the PIPn regulation module within the NH2-terminal region of the same subunit had been silenced. These results, summarized in Fig. 8E, are consistent with intersubunit coupling between NH2- and COOH-terminal regions that controls the PIPn sensitivity of CNGA3 channels.
Fig. 8.
Tandem CNGA3 heterodimers containing L633P reveal intersubunit N-C terminal interaction controlling PIPn sensitivity. Four scenarios illustrate how intersubunit N-C terminal interactions control PIPn regulation of CNGA3 subunits, respectively. These PIPn regulation modules can be silenced (red) or activated (green) owing to changes in intersubunit interactions. Representative cGMP dose-response curves before and after 10 μM PIP2 (gray) are shown for activation of each channel made up of these dimers. A: tandem linkage of wild-type A3 subunits. B: channels formed by tandem A3//A3 L633P dimers. The Hill parameters for representative A3//A3 L633P channels were as follows: before PIP2: K1/2,cGMP = 5.72 μM, h = 1.6; after PIP2: K1/2,cGMP = 13.3 μM, h = 1.6. C: A3 N7R-A//A3 L633P tandem dimers. D: channels formed by A3//A3 N7R-A, L633P tandem dimers. The Hill parameters for representative A3//A3 N7R-A, L633P channels were as follows: before PIP2: K1/2,cGMP = 6.58 μM, h = 1.6; after PIP2: K1/2,cGMP = 13.1 μM, h = 1.8. E: summary showing the percent increase in K1/2,cGMP after 10 μM PIP2 for tandem dimer constructs, as in A–D, or after coexpression of the respective constituent monomers (n = 4). *P < 0.05 compared with A3//A3 dimer channels.
The interpretation of tandem dimer experiments requires some caution since these approaches can be sensitive to potential artifacts due to aberrant assembly (35). Potential artifacts include exclusion of the second tandem dimer subunit from the channel tetramer, or possible linker proteolysis. For A3//A3 N7R-A, L633P dimers, since each individual subunit of the dimer alone generates PIPn-insensitive channels, neither of these potential assembly artifacts can explain the formation of PIPn-sensitive channels. In support of our interpretation of intersubunit N-C interactions, we found that coexpression of the respective PIPn-insensitive A3 wild-type and A3 N7R-A, L633P monomer constituents also produced channels that reconstituted PIPn inhibition of apparent cGMP affinity, exhibiting a 62.2 ± 12.5% (n = 4) increase in K1/2,cGMP (Fig. 8E). Since A3 wild-type subunits produce functional channels more efficiently than A3 N7R-A, L633P subunits (Table 1), a 1:5 ratio of the respective cRNAs was injected for coexpression experiments. Assuming a binominal distribution of channel species via free assembly of the monomer constituents, an average of approximately one favorable intersubunit N-C interaction per channel is expected. In contrast to PIPn-insensitive A3 N7R-A//A3 L633P dimers, coexpression of the respective A3 N7R-A and A3 L633P monomer constituents produced some channels that were PIPn sensitive, exhibiting a 48.3 ± 9.2% increase in K1/2,cGMP (n = 4) (Fig. 8E). This difference in PIPn sensitivity observed between A3 N7R-A//A3 L633P dimers and coexpression A3-N7R-A and A3-L633P monomers again suggests that the tandem dimer configuration successfully constrains the subunit arrangement for CNGA3 tetramers. Taken together, these results validate our interpretation of the tandem dimer experiments described above and are consistent with intersubunit interactions controlling PIPn sensitivity of CNGA3 channels.
DISCUSSION
Characterization of the achromatopsia-associated mutation L633P in human CNGA3 has provided mechanistic insight into cone CNG channel regulation by PIPn and the potentiation of PIPn sensitivity by L633P. We propose a model where L633P disrupts an interaction between NH2- and COOH-terminal cytoplasmic domains of adjacent A3 subunits, which serves to unmask and activate the PIPn interaction module located within the NH2-terminal domain of A3. The results suggest that in addition to a role in assembly of CNG channel subunits, the CLZ domain of CNGA3 also may help control PIPn regulation of the channel by participating in intersubunit interactions. Similarly, assembly of A3 with B3 subunits likely alters some of these N-C interactions (by substitution), tuning the channel's PIPn sensitivity. L633P still can potentiate the PIPn sensitivity of heteromeric A3 + B3 channels, possibly due to effects on A3-A3 interactions within the tetramer.
The CLZ domain of CNG channels has been proposed to play an important role in channel assembly. Previous studies by Zhong et al. (68) have shown that mutation of hydrophobic heptad-repeat residues within the CLZ domain of CNGA3 decreased cGMP-activated current density in HEK 293T cells. In addition, L633A mutation attenuated l-cis-diltiazem inhibition and the Imax,cAMP/Imax,cGMP ratio for CNGA3 + CNGB1 channels, indicating that the CLZ domain is critical for selective assembly of heteromeric channels (68). Consistent with these studies, we have observed that the current density for L633P-containing channels is similarly decreased and that formation of heteromeric CNGA3 + CNGB3 channels is slightly impaired by L633P when channels are expressed in oocytes. However, we also observed changes in the gating properties of L633P channels that were not reported with CLZ deletions or other mutations (68). This is not surprising because radically disrupting the α-helical structure of the CLZ domain may have a different effect on channel gating compared with deleting the CLZ domain. Furthermore, we found that mutating a cluster of hydrophobic residues within the distal part of the CLZ domain (L662A, L665A, and V669A) similarly potentiated the PIPn sensitivity of the channel; the K1/2,cGMP was shifted from 5.67 ± 0.21 μM to 10.90 ± 0.93 μM by PIP2 (92.4 ± 11.2% increase; n = 4). Together, these data suggest that the structural integrity of the entire CLZ domain might be necessary for proper PIPn regulation of the channel.
We have used multiple approaches that together suggest N-C interactions help control PIPn regulation of CNGA3 channels. However, we have not yet localized specific residues necessary for this interaction. We investigated whether possible salt bridges between critical subdomains might contribute to interactions between NH2- and COOH-terminal regions. However, several charge reversal mutations within COOH-terminal region between 624 and 635 (E627R, E628R, E631R) (Fig. 4C), as well as charge reversals within the region surrounding P99 and A105 in the NH2-terminal domain (R101E, R103E and K108E), produced only subtle effects on initial K1/2,cGMP and PIPn sensitivity of A3 channels (for A3 R101E, R103E, K108E channels, the percent increase of K1/2,cGMP after 1 μM PIP3 was 28.8 ± 4.0%). Besides salt bridges, other types of protein-protein interfaces, such as hydrophobic interactions, may play a role. Alternatively, indirect interactions mediated by other regions of the channel or by unknown channel-associated proteins may support N-C coupling in CNGA3 channels.
Intersubunit N-C interactions also are characteristic of other CNG channel types. In rod CNG channels, the NH2-terminal region of CNGB1 has been shown to interact with the CLZ domain of CNGA1; Ca2+-CaM binding to the NH2-terminal calmodulin-binding module within CNGB1 disrupts this intersubunit interaction (56). A recent study showed that coexpression of CNGA1-ΔCLZ with CNGB1-ΔN subunits generated channels with a mixture of channel subunit stoichiometries (51). Interestingly, coexpression of CNGA1-ΔCLZ with intact CNGB1 subunits basically eliminated all functional channel expression in Xenopus oocytes, whereas coexpression of CNGA1-ΔCLZ with CNGB1-ΔN subunits restored expression, consistent with an intersubunit interaction between CNGA1 and the NH2-terminal region of CNGB1 (51). In addition, intersubunit disulfide bonds can be formed between endogenous cysteines within NH2-terminal and C-linker regions of CNGA1 (49). Furthermore, the NH2-terminal GARP domain of CNGB1 was found to act as gating inhibitor of rod channels via direct protein-protein interactions with CNGA1 (36). In CNGA2 channels, Ca2+-CaM can bind to an NH2-terminal site, which disrupts an autoexcitatory interdomain interaction between the NH2- and COOH-terminal regions of adjacent CNGA2 subunits and downregulates channel activity (61, 67). Furthermore, N-C interactions have been observed in other homologous CNBD-containing channels. In human ether-á-go-go-related gene (hERG) potassium channels, intersubunit interactions between the NH2-terminal “EAG domain” and the COOH-terminal CNB-homology domain have been shown to regulate the slow deactivation of the channel (20, 37). CNG channels belong to the superfamily of voltage-gated cation channels (27). Plausible N-C interactions are consistent with the fundamental structural organization of voltage-gated sodium and calcium channels having N-C linkages between pseudo subunits, and with the successful generation of functional channels using concatenated cDNA constructs for tandem potassium and CNG channel subunits (42, 58). Furthermore, at a different structural level, the potential for intersubunit coupling is suggested by the structure of voltage-gated potassium channels, which show the offset voltage-sensor domain (S1–S4) of one subunit in close proximity to the pore domain and contiguous COOH-terminal module of the adjacent subunit (33). Together with the intersubunit interactions of CNGA3 channels described in this paper, we propose that N-C interdomain interactions may be a general feature for the family of CNBD-containing ion channels.
In addition to influencing N-C coupling, L633P mutation might exert an effect on the CNBD of CNGA3. Previous work has demonstrated that specific residues within the αC helix of the CNBD are critical for ligand selectivity of CNG channels and hyperpolarization-activated cyclic nucleotide-regulated (HCN) channels (11, 60, 70). In HCN2 channels, the αC helix undergoes a conformational rearrangement and is stabilized after binding of the ligand to the CNBD (55). Because of the close proximity of the CNBD and CLZ domains, disruption of the CLZ domain by L633P or other mutations may have an effect on the αC helix of the CNBD. The PIPn-dependent increase in cAMP efficacy for homomeric A3 channels was eliminated by L633P. It is possible that PIPn could have an effect on the CLZ domain (directly or indirectly) and therefore influence the αC helix of CNBD and ultimately alter the coupling of cGMP or cAMP binding to channel gating. L633P may abolish either a potential PIPn interaction with the COOH-terminal region of A3 or the ability of the COOH-terminal region to communicate the PIPn-binding event elsewhere in the channel to the CNBD.
Overall, our results show that the achromatopsia-associated mutation L633P produces a gain-of-function change for gating of CNG channels, increasing the apparent affinity for cGMP. However, L633P produces a loss-of-function effect regarding functional expression level (current density). In addition, L633P results in abnormal channel regulation by PIPn. Phosphoinositide metabolism is very active in retinal photoreceptors. For example, light activation of the insulin receptor/phosphoinositide 3-kinase (PI3K)/Akt pathway generates PIP3 in photoreceptors (31, 44). Downregulation of PI3K/Akt pathway in cone photoreceptor results in cone degeneration (26). Moreover, circadian phase-dependent regulation of cone CNG channels by somatostatin is mediated by phospholipase C (PLC), which cleaves PIP2 within the membrane (6). Therefore, light and/or circadian-dependent modulation of photoreceptor CNG channels may be mediated in part by phosphoinositides. Enhanced PIPn sensitivity of the cone channel due to L633P could potentially impair the ability of the channels to appropriately adjust ligand sensitivity in response to light or circadian inputs.
GRANTS
This research was supported by National Institutes of Health National Eye Institute Grant EY-12836 (to M. D. Varnum).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.D. performed the experiments; G.D. and M.D.V. analyzed the data; G.D. and M.D.V. interpreted the results of the experiments; G.D. and M.D.V. prepared the figures; G.D. drafted the manuscript; G.D. and M.D.V. edited and revised the manuscript; G.D. and M.D.V. approved the final version of the manuscript; M.D.V. conception and design of the research.
ACKNOWLEDGMENTS
We are grateful to Elizabeth Rich for excellent technical support and for performing pilot experiments, Dr. K.-W. Yau for sharing the human CNGA3 cDNA clone, and Drs. R. Lane Brown, Pete Meighan, Starla Meighan, and Tshering Sherpa for helpful comments.
Footnotes
This article is the topic of an Editorial Focus by Donna L. Cioffi and Thomas C. Rich (6a).
REFERENCES
- 1. Biel M, Michalakis S. Function and dysfunction of CNG channels: insights from channelopathies and mouse models. Mol Neurobiol 35: 266–277, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bönigk W, Altenhofen W, Müller F, Dose A, Illing M, Molday RS, Kaupp UB. Rod and cone photoreceptor cells express distinct genes for cGMP-gated channels. Neuron 10: 865–877, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Brady JD, Rich ED, Martens JR, Karpen JW, Varnum MD, Brown RL. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 103: 15635–15640, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bright SR, Rich ED, Varnum MD. Regulation of human cone cyclic nucleotide-gated channels by endogenous phospholipids and exogenously applied phosphatidylinositol 3,4,5-trisphosphate. Mol Pharmacol 71: 176–183, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron 48: 387–401, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chen SK, Ko GYP, Dryer SE. Somatostatin peptides produce multiple effects on gating properties of native cone photoreceptor cGMP-gated channels that depend on circadian phase and previous illumination. J Neurosci 27: 12168–12175, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a. Cioffi DL, Rich TC. Feedback regulation of cone cyclic nucleotide channels by phosphoinositides. Focus on “CNGA3 achromatopsia-associated mutation potentiates the phosphoinositide sensitivity of cone photoreceptor CNG channels by altering intersubunit interactions.” Am J Physiol Cell Physiol (May 15, 2013). 10.1152/ajpcell.00136.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Craven KB, Olivier NB, Zagotta WN. C-terminal movement during gating in cyclic nucleotide-modulated channels. J Biol Chem 283: 14728–14738, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a. Dai G, Peng C, Liu C, Varnum MD. Two structural components in CNGA3 support regulation of cone CNG channels by phosphoinositides. J Gen Physiol 141: 413–430, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding XQ, Matveev A, Singh A, Komori N, Matsumoto H. Biochemical characterization of cone cyclic nucleotide-gated (CNG) channel using the infrared fluorescence detection system. Adv Exp Med Biol 723: 769–775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev 81: 117–151, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313: 310–313, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Flynn GE, Black KD, Islas LD, Sankaran B, Zagotta WN. Structure and rearrangements in the carboxy-terminal region of SpIH channels. Structure 15: 671–682, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flynn GE, Johnson JP, Jr, Zagotta WN. Cyclic nucleotide-gated channels: shedding light on the opening of a channel pore. Nat Rev Neurosci 2: 643–651, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci 8: 921–934, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gerstner A, Zong X, Hofmann F, Biel M. Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina. J Neurosci 20: 1324–1332, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golebiewska U, Nyako M, Woturski W, Zaitseva I, McLaughlin S. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol Biol Cell 19: 1663–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon SE, Downing-Park J, Zimmerman AL. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol 486: 533–546, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goto-Omoto S, Hayashi T, Gekka T, Kubo A, Takeuchi T, Kitahara K. Compound heterozygous CNGA3 mutations (R436W, L633P) in a Japanese patient with congenital achromatopsia. Vis Neurosci 23: 395–402, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Grunwald ME, Zhong H, Lai J, Yau KW. Molecular determinants of the modulation of cyclic nucleotide-activated channels by calmodulin. Proc Natl Acad Sci USA 96: 13444–13449, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guido ME, Garbarino Pico E, Caputto BL. Circadian regulation of phospholipid metabolism in retinal photoreceptors and ganglion cells. J Neurochem 76: 835–845, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Gustina AS, Trudeau MC. hERG potassium channel gating is mediated by N- and C-terminal region interactions. J Gen Physiol 137: 315–325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hackos DH, Korenbrot JI. Calcium modulation of ligand affinity in the cyclic GMP-gated ion channels of cone photoreceptors. J Gen Physiol 110: 515–528, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron 30: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Haynes LW, Stotz SC. Modulation of rod, but not cone, cGMP-gated photoreceptor channels by calcium-calmodulin. Vis Neurosci 14: 233–239, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Hilgemann DW. Local PIP2 signals: when, where, how? Pflügers Arch 455: 55–67, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361: 76–79, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Ivanovic I, Anderson RE, Le YZ, Fliesler SJ, Sherry DM, Rajala RV. Deletion of the p85alpha regulatory subunit of phosphoinositide 3-kinase in cone photoreceptor cells results in cone photoreceptor degeneration. Invest Ophthalmol Vis Sci 52: 3775–3783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jan LY, Jan YN. A superfamily of ion channels. Nature 345: 672, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Kaupp UB, Niidome T, Tanabe T, Terada S, Bönigk W, Stühmer W, Cook NJ, Kangawa K, Matsuo H, Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature 342: 762–766, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Kusch J, Zimmer T, Holschuh J, Biskup C, Schulz E, Nache V, Benndorf K. Role of the S4-S5 linker in CNG channel activation. Biophys J 99: 2488–2496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li G, Rajala A, Wiechmann AF, Anderson RE, Rajala RVS. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J Neurochem 107: 1382–1397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu C, Varnum MD. Functional consequences of progressive cone dystrophy-associated mutations in the human cone photoreceptor cyclic nucleotide-gated channel CNGA3 subunit. Am J Physiol Cell Physiol 289: C187–C198, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309: 897–903, 2005 [DOI] [PubMed] [Google Scholar]
- 34. MacArthur MW, Thornton JM. Influence of proline residues on protein conformation. J Mol Biol 218: 397–412, 1991 [DOI] [PubMed] [Google Scholar]
- 35. McCormack K, Lin L, Iverson LE, Tanouye MA, Sigworth FJ. Tandem linkage of Shaker K+ channel subunits does not ensure the stoichiometry of expressed channels. Biophys J 63: 1406–1411, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michalakis S, Zong X, Becirovic E, Hammelmann V, Wein T, Wanner KT, Biel M. The glutamic acid-rich protein is a gating inhibitor of cyclic nucleotide-gated channels. J Neurosci 31: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muskett FW, Thouta S, Thomson SJ, Bowen A, Stansfeld PJ, Mitcheson JS. Mechanistic insight into human ether-à-go-go-related gene (hERG) K+ channel deactivation gating from the solution structure of the EAG domain. J Biol Chem 286: 6184–6191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson DL, Cox MM. Lehninger Principles of Biochemistry (5th ed) New York: W. H. Freeman, 2008 [Google Scholar]
- 39. Nishiguchi KM, Sandberg MA, Gorji N, Berson EL, Dryja TP. Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Hum Mutat 25: 248–258, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Peng C, Rich ED, Thor CA, Varnum MD. Functionally important calmodulin-binding sites in both NH2- and COOH-terminal regions of the cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit. J Biol Chem 278: 24617–24623, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Peng C, Rich ED, Varnum MD. Achromatopsia-associated mutation in the human cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit alters the ligand sensitivity and pore properties of heteromeric channels. J Biol Chem 278: 34533–34540, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron 42: 401–410, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Pugh EN, Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr Opin Neurobiol 9: 410–418, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Rajala RVS. Phosphoinositide 3-kinase signaling in the vertebrate retina. J Lipid Res 51: 4–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rattner A, Sun H, Nathans J. Molecular genetics of human retinal disease. Annu Rev Genet 33: 89–131, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Rebrik TI, Botchkina I, Arshavsky VY, Craft CM, Korenbrot JI. CNG-modulin: a novel Ca-dependent modulator of ligand sensitivity in cone photoreceptor cGMP-gated ion channels. J Neurosci 32: 3142–3153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rebrik TI, Korenbrot JI. In intact mammalian photoreceptors, Ca2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods. J Gen Physiol 123: 63–75, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reuter P, Koeppen K, Ladewig T, Kohl S, Baumann B, Wissinger B. Mutations in CNGA3 impair trafficking or function of cone cyclic nucleotide-gated channels, resulting in achromatopsia. Hum Mutat 29: 1228–1236, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Rosenbaum T, Gordon SE. Dissecting intersubunit contacts in cyclic nucleotide-gated ion channels. Neuron 33: 703–713, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Sakmann B, Neher E. (Eds.). Single-Channel Recording (2nd ed). New York: Plenum, 1995 [Google Scholar]
- 51. Shuart NG, Haitin Y, Camp SS, Black KD, Zagotta WN. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat Commun 2: 457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simunovic MP, Moore AT. The cone dystrophies. Eye (Lond) 12: 553–565, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sundin OH, Yang JM, Li Y, Zhu D, Hurd JN, Mitchell TN, Silva ED, Maumenee IH. Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet 25: 289–293, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Taraska JW, Puljung MC, Olivier NB, Flynn GE, Zagotta WN. Mapping the structure and conformational movements of proteins with transition metal ion FRET. Nat Methods 6: 532–537, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trudeau MC, Zagotta WN. Mechanism of calcium/calmodulin inhibition of rod cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 99: 8424–8429, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trudeau MC, Zagotta WN. Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels. J Biol Chem 278: 18705–18708, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Tytgat J, Hess P. Evidence for cooperative interactions in potassium channel gating. Nature 359: 420–423, 1992 [DOI] [PubMed] [Google Scholar]
- 59. Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Varnum MD, Black KD, Zagotta WN. Molecular mechanism for ligand discrimination of cyclic nucleotide-gated channels. Neuron 15: 619–625, 1995 [DOI] [PubMed] [Google Scholar]
- 61. Varnum MD, Zagotta WN. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science 278: 110–113, 1997 [DOI] [PubMed] [Google Scholar]
- 62. Wall-Lacelle S, Hossain MI, Sauvé R, Blunck R, Parent L. Double mutant cycle analysis identified a critical leucine residue in the IIS4S5 linker for the activation of the Ca(V)2.3 calcium channel. J Biol Chem 286: 27197–27205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wissinger B, Gamer D, Jägle H, Giorda R, Marx T, Mayer S, Tippmann S, Broghammer M, Jurklies B, Rosenberg T, Jacobson SG, Sener EC, Tatlipinar S, Hoyng CB, Castellan C, Bitoun P, Andreasson S, Rudolph G, Kellner U, Lorenz B, Wolff G, Verellen-Dumoulin C, Schwartz M, Cremers FP, Apfelstedt-Sylla E, Zrenner E, Salati R, Sharpe LT, Kohl S. CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet 69: 722–737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Womack KB, Gordon SE, He F, Wensel TG, Lu CC, Hilgemann DW. Do phosphatidylinositides modulate vertebrate phototransduction? J Neurosci 20: 2792–2799, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu WP, Grunwald ME, Yau KW. Molecular cloning, functional expression and chromosomal localization of a human homolog of the cyclic nucleotide-gated ion channel of retinal cone photoreceptors. FEBS Lett 393: 211–215, 1996 [DOI] [PubMed] [Google Scholar]
- 66. Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36: 891–896, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Zheng J, Varnum MD, Zagotta WN. Disruption of an intersubunit interaction underlies Ca2+-calmodulin modulation of cyclic nucleotide-gated channels. J Neurosci 23: 8167–8175, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhong H, Lai J, Yau KW. Selective heteromeric assembly of cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 100: 5509–5513, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 420: 193–198, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou L, Siegelbaum SA. Gating of HCN channels by cyclic nucleotides: residue contacts that underlie ligand binding, selectivity, and efficacy. Structure 15: 655–670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]