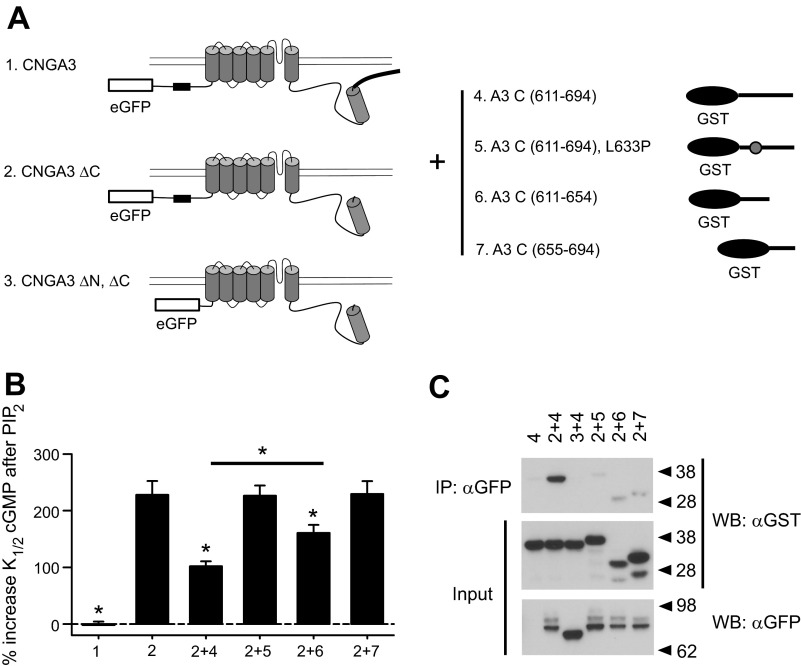
Fig. 7.
Coexpression of soluble CNGA3 COOH-terminal fragments supports interdomain NH2-COOH (N-C) interactions regulating PIPn sensitivity. A: cartoon showing the constructs used for coexpression experiments. CNGA3ΔC is equivalent to 613X, and CNGA3 ΔN, ΔC combines NH2-terminal Δ2–107 and COOH-terminal Δ613–694 truncations. The NH2-terminal PIPn regulation site is shown as a black box. eGFP, enhanced green fluorescent protein. B: summary showing effects of PIP2 on A3ΔC channels coexpressed with soluble A3 COOH-terminal fragments. Coexpression of A3 COOH-terminal fragments representing the region after the CNBD (aa 611–694) with A3ΔC channels significantly attenuated the increase in K1/2,cGMP after PIP2 application. However, addition of L633P to this fragment prevented this effect. The proximal A3 COOH-terminal fragment (611–654) exerted a similar effect to the full-length fragment while the more distal A3 COOH-terminal fragment (655–694) failed to attenuate the PIP2-induced increase in K1/2,cGMP for A3 ΔC channels; *P < 0.05 compared with A3ΔC channels alone. C: immunoblots are shown for coimmunoprecipitation of GST-tagged COOH-terminal fragments with eGFP-tagged A3ΔC or A3ΔNΔC channels. A3 C(611–694) was able to bind to A3ΔC (2 + 4), but not to A3ΔN,ΔC (3 + 4) in vitro. L633P prevented binding of A3 C(611–694) to A3ΔC channels (2 + 5). Both of the smaller A3 COOH-terminal fragments showed weak binding (2 + 6, 2 + 7). Approximate molecular weight markers are indicated to the right of the blots. The slower gel migration of the L633P-containing fragment (5) likely reflects a less compact structure compared with the wild-type fragment. Lower-molecular-weight bands for fragments 6 and 7 represent minor proteolytic products that retain the GST tag. Note that the top molecular weight bands shown in the bottom blot represent glycosylated CNGA3 subunits.