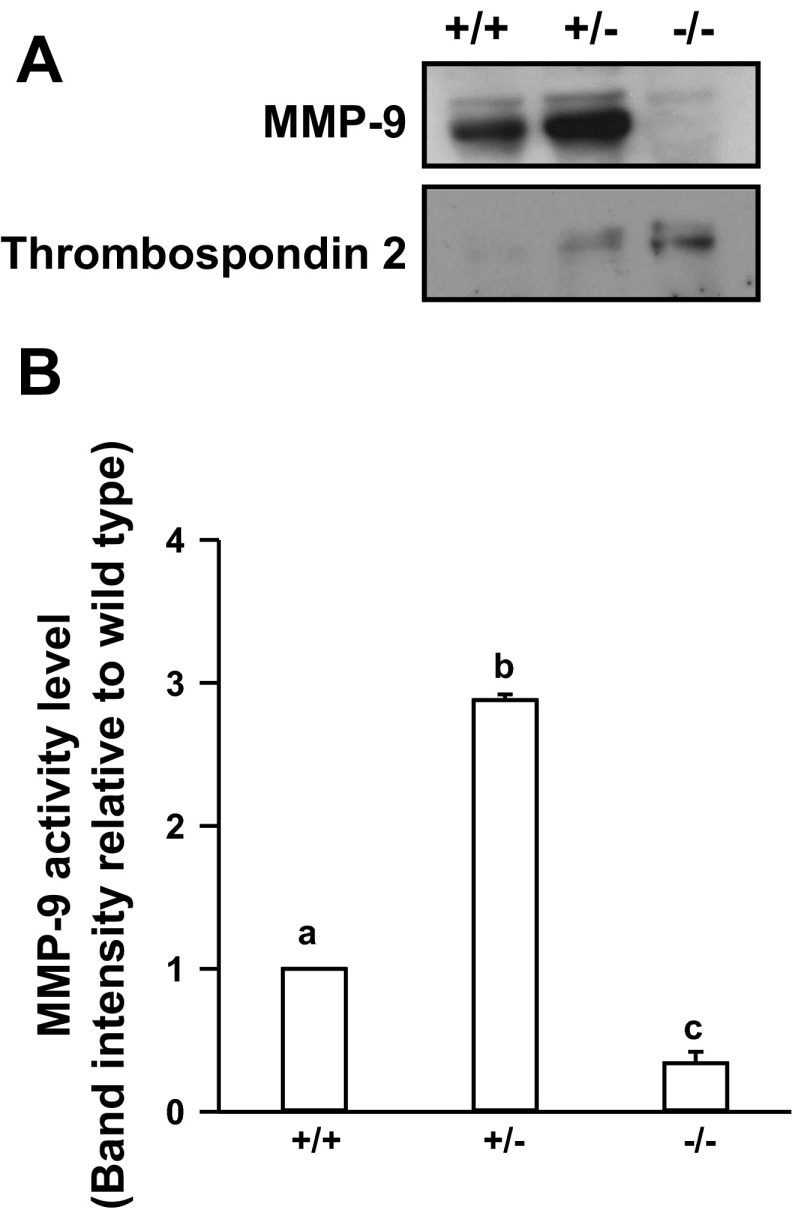
Fig. 1.
Matrix metalloproteinase-9 (MMP-9) protein and gelatinase activity are reduced in the distal-less 3-null (Dlx3−/−) mouse placenta. A: wild-type (Dlx3+/+), Dlx3 heterozygous (Dlx3+/−), and Dlx3−/− placental explants (n = 4–6/genotype) were cultured for 4 h and media were collected. Western blot analysis was used to determine secreted MMP-9 and thrombospondin 2 protein abundance in the Dlx3−/− compared with Dlx3+/+. B: zymography studies were performed to determine the amount of secreted MMP-9 gelatinase activity from the explant study. Gels were scanned and densiotometry measurements were obtained to determine the level of gelatinase activity corresponding to a band consistent with the molecular weight of MMP-9. All studies were replicated on at least 3 separate occasions with equivalent results. Bars with varying letters differ, P ≤ 0.05.