Abstract
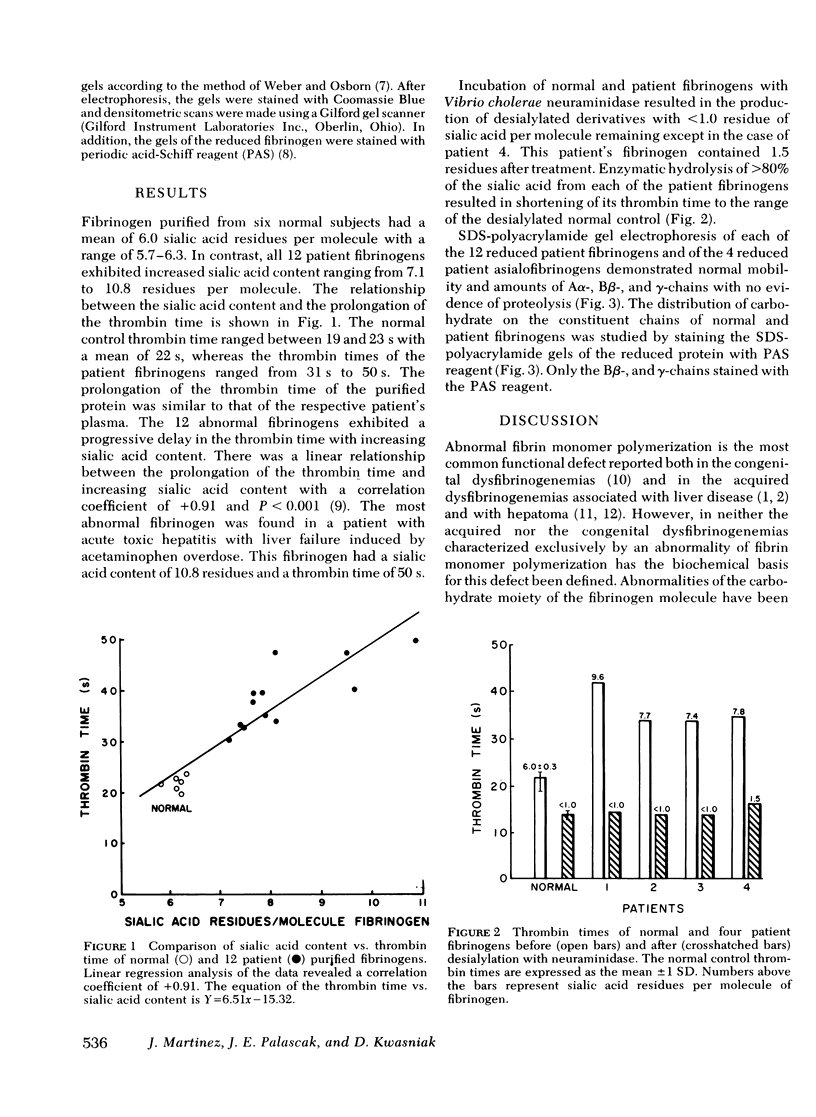
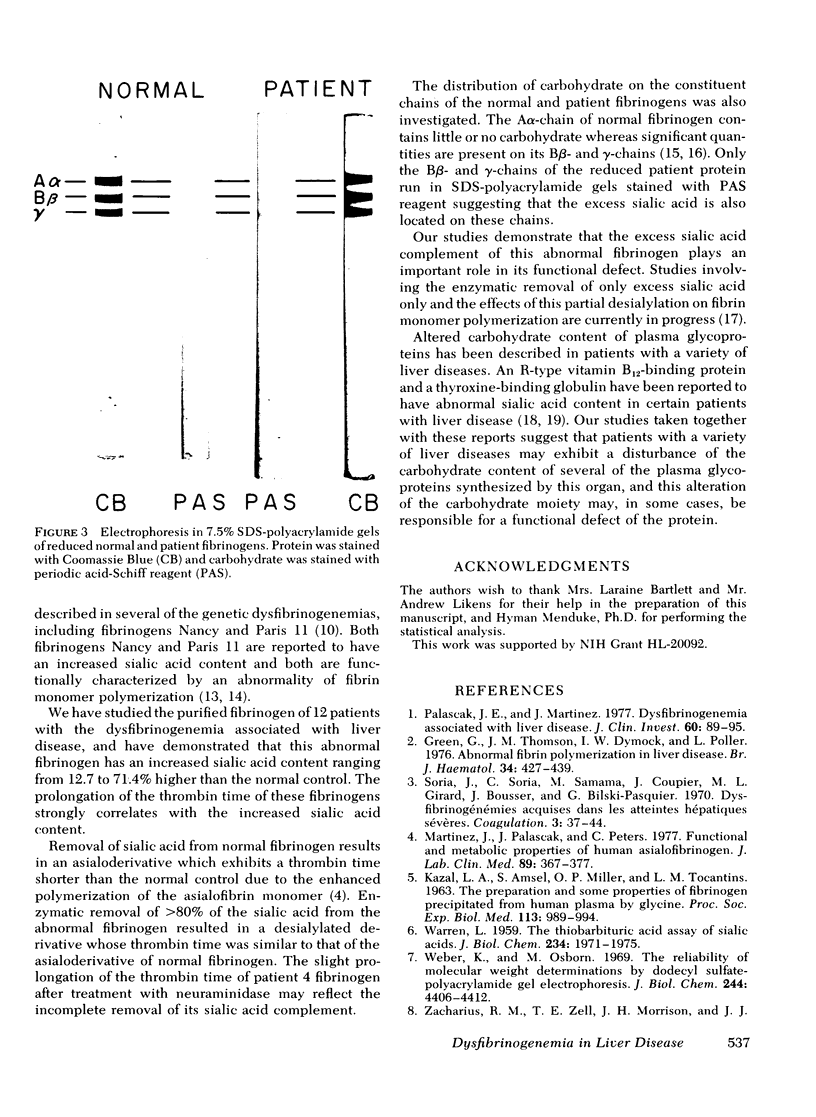
To evaluate the possibility that the carbohydrate composition of fibrinogen may be altered in the dysfibrinogenemia associated with liver disease, we studied the sialic acid content of purified fibrinogen from 12 patients with liver disease and its relationship to the prolongation of the thrombin time. Purified fibrinogen showed that Aalpha-, Bbeta-, and gamma-chains when reduced and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and exhibited prolongation of the thrombin time similar to that of the plasma from which it was derived. Sialic acid content of the purified fibrinogen ranged from 12.7 to 71.4% higher in patient fibrinogens when compared to normal controls. A progressive delay in thrombin time was associated with increasing sialic acid content of the patient fibrinogen. Enzymatic removal of sialic acid from four of the abnormal fibrinogens resulted in a shortening of their thrombin times to the range of the desialylated normal control. Periodic acid-Schiff reagent stained only the Bbeta- and gamma-chains of the reduced patient fibrinogens after sodium dodecyl sulfate-polyacrylamide gel electrophoresis suggesting that the excess sialic acid is located on these two chains. These studies demonstrate a biochemical alteration of the functionally abnormal fibrinogen found in some patients with liver disease, and indicate that the excess sialic acid plays an important role in the functional defect of this protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R. L., Waxman S., Gilbert H. S., Mehlman C. S., Allen R. H. Isolation and characterization of a novel vitamin B12-binding protein associated with hepatocellular carcinoma. J Clin Invest. 1975 Nov;56(5):1262–1270. doi: 10.1172/JCI108202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G., Thomson J. M., Dymock I. W., Poller L. Abnormal fibrin polymerization in liver disease. Br J Haematol. 1976 Nov;34(3):427–439. doi: 10.1111/j.1365-2141.1976.tb03589.x. [DOI] [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Green A. M., Pensky J., Williams S., Zinn A., Carlson D. M. Measurement of circulating desialylated glycoproteins and correlation with hepatocellular damage. J Clin Invest. 1974 Sep;54(3):555–562. doi: 10.1172/JCI107792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Palascak J., Peters C. Functional and metabolic properties of human asialofibrinogen. J Lab Clin Med. 1977 Feb;89(2):367–377. [PubMed] [Google Scholar]
- Mester L., Szabados L. Différences constitutionnelles et fonctionnelles entre les fragments glucidiques du fibrinogène humain normal et d'un fibrinogène humain anormal. Bull Soc Chim Biol (Paris) 1968;50(12):2561–2566. [PubMed] [Google Scholar]
- Palascak J. E., Martinez J. Dysfibrinogenemia associated with liver disease. J Clin Invest. 1977 Jul;60(1):89–95. doi: 10.1172/JCI108773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972 Feb 10;247(3):636–645. [PubMed] [Google Scholar]
- Ratnoff O. D., Forman W. B. Criteria for the differentiation of dysfibrinogenemic states. Semin Hematol. 1976 Apr;13(2):141–157. [PubMed] [Google Scholar]
- Streiff F., Alexandre P., Vigneron C., Soria J., Soria C., Mester L. Un nouveau Ccas d'anomalie constitutionnelle et familiale du Fibrinogène sans Diathese hémorragique. Thromb Diath Haemorrh. 1971 Dec 31;26(3):565–576. [PubMed] [Google Scholar]
- Verhaeghe R., van Damme B., Molla A., Vermylen J. Dysfibrinogenaemia associated with primary hepatoma. Scand J Haematol. 1972;9(5):451–458. doi: 10.1111/j.1600-0609.1972.tb00968.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- von Felten A., Straub P. W., Frick P. G. Dysfibrinogenemia in a patient with primary hepatoma. First observation of an acquired abnormality of fibrin monomer aggregation. N Engl J Med. 1969 Feb 20;280(8):405–409. doi: 10.1056/NEJM196902202800802. [DOI] [PubMed] [Google Scholar]



