Fig. 5.
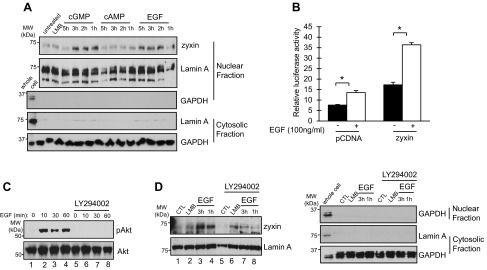
Zyxin translocates to the nucleus in response to cGMP and EGF. A: mIMCD3 cells were treated with 8-bromo-cGMP (100 nM), dibutyryl-cAMP (100 nM), or EGF (100 ng/ml) for the indicated time periods. Nuclear extracts were subjected to Western blotting with anti-zyxin and anti-lamin A antibodies. LMB, leptomycin B. B: stably transfected mIMCD3 cells containing a chromosomally integrated Pkhd1-Gal4-VP16 transgene were cotransfected with either pcDNA or pCMV-MYC-zyxin and a Gal4-luciferase reporter plasmid. After 24 h, the cells were treated with EGF (100 ng/ml) for 16 h (open bars) or vehicle (closed bars). Luciferase activity was measured 40 h after transfection. C: mIMCD3 cells were treated with EGF (100 ng/ml) for the indicated time period in the presence or absence of LY294002 (50 μM). Cell lysates were subjected to Western blotting with anti-Akt and anti-phospho-Akt antibodies. D: mIMCD3 cells were treated with EGF in the presence or absence of LY294002 (50 μM). Accumulation of zyxin in nuclear extracts was measured by Western blotting with an anti-zyxin antibody. Lamin A was used as a nuclear marker protein. Anti-GAPDH and anti-lamin A antibodies were used as a marker for the purity of cytosolic and nuclear fractions (A and D). Results represent the means of 3 independent experiments. Error bars indicate SD. *P < 0.05.
