Abstract
Inconsistencies between species has stunted the progress of developing new analgesics. To increase the success of translating results between species, improved comparable models are required. Twelve rats received rectal balloon distensions on 2 different days separated by 24.3 (SD 24.6) days. Rectal balloon distensions were also performed in 18 humans (mean age: 34 yr; range: 21–56 yr; 12 men) on two separate occasions, separated by 9.3 (SD 5.5) days. In rats, cerebral evoked potentials (CEPs) were recorded by use of implanted skull-electrodes to distension pressure of 80 mmHg. In humans surface electrodes and individualized pressure, corresponding to pain detection threshold, were used. Comparison of morphology was assessed by wavelet analysis. Within- and between-day reproducibility was assessed in terms of latencies, amplitudes, and frequency content. In rats CEPs showed triphasic morphology. No differences in latencies, amplitudes, and power distribution were seen within or between days (all P ≥ 0.5). Peak-to-peak amplitude between the first positive and negative potential were the most reproducible characteristic within and between days (evaluated by intraclass correlation coefficients, ICC) (ICC = 0.99 and ICC = 9.98, respectively). In humans CEPs showed a triphasic morphology. No differences in latencies, amplitudes, or power distribution were seen within or between days (all P ≥ 0.2). Latency to the second negative potential (ICC = 0.98) and the second positive potential (ICC = 0.95) was the most reproducible characteristic within and between days. A unique and reliable translational platform was established assessing visceral sensitivity in rats and humans, which may improve the translational process of developing new drugs targeting visceral pain.
Keywords: translation, mechanical rectal distension, evoked potentials, reproducibility
development of new analgesics is very costly. A main contributing factor to this cost is thought to be a result of high attrition rates (27). One reason for these high attrition rates could be inconsistencies between species, resulting in inhibited progress of developing new analgesics. The relatively inadequate predictive value of preclinical animal models used to test pharmacological compounds before using them in human clinical trials increases the demand of objective translational comparable models.
Because the underlying pathophysiology remains unknown in many pain and central nervous system disorders, the construct validity of animal models may be limited and unlikely to reflect the clinical picture in humans that they attempt to model. Hence, an increased understanding of the underlying pathophysiological mechanisms will enhance the possibility of creating animal models with better construct validity. However, this remains challenging and an ideal model needs to be accurate, responsive, sensitive to therapy, and better than existing models.
An alternative method to increase the predictive values of animal models would be to establish translational comparable experimental methods. These should include accurate objective test stimuli and responses that are reliably assessed in animals and humans. Colorectal distension has been used in experimental pain models in both animal and humans and has previously proven to be relevant for the visceral sensory system (18, 29, 37). In animals, colorectal distension induces contraction of the abdominal musculature, the so-called visceromotor response. This model has widely been used to evaluate the analgesic effect of drugs in animals (39, 46). However, a major limitation of this response is that it is mediated through a brain stem reflex and does not assess the supraspinal aspect of evoked sensation (41). A consequence of this limitation could result in failure of transferring analgesic effects seen in rat visceromotor response studies to human experiments (12, 17). However, the visceromotor response model has shown good predictive value in numerous studies (4, 24, 51, 55). In humans, patients suffering from irritable bowel syndrome report lower pain detection threshold to colorectal distension than healthy volunteers (29, 35), indicating that the model is sensitive to underlying pathophysiology.
An improved translational model includes the use of cerebral evoked potentials (CEPs) to objectively assess the brain response to colorectal distension in animals and humans (16, 19, 20, 33, 37, 38). In previous studies, electrically elicited CEPs from the gut have been favored, compared with balloon distension, because this stimulus is easier to control with respect to localization, onset, and duration. However, electrical stimulation bypasses all receptors, causing a direct depolarization of axons, and is considered an unnatural nonphysiological stimulus that bears little relevance to the pain and physiological mechanisms occurring in patients suffering from visceral pain. In contrast, although rapid colorectal distension is challenging to control, it has the advantage of stimulating mechanoreceptors in a more physiological way, resembling normal gut distension.
Recently, a novel rat model combining CEPs and colorectal distension has been established in conscious rats. The model was used to show an association between stimulation intensity and amplitude of the CEPs (23). Furthermore, administration of lidocaine was shown to diminish the amplitude of CEPs, indicating that the model is sensitive to peripheral blockade of sensory receptors. Interestingly, nonnoxious stimulations also resulted in CEPs, suggesting that the recorded CEPs do not represent a specific response to noxious sensation, but rather a response to colorectal sensation in general.
There have been several previous attempts to establish accurate and reproducible electrophysiological models using rectal mechanical CEPs in humans (7, 16, 18, 32, 33). However, these efforts were limited by insufficient mechanical pumps and recording techniques. CEPs were distorted by reduced signal-to-noise ratio, and therefore electrical stimulation has been considered superior. However, technological improvements and advanced signal analysis tools have renewed the interests in the reproducibility of such a physiologically relevant model, since it may provide a resource-saving approach to study basic visceral sensation as well as pharmacological interventions.
We hypothesized that an accurate, reproducible, and objective model comparing the response to rectal distensions in rats and humans could be established. The establishment of such a model may facilitate translation of results from preclinical drug development to efficacy in clinical human settings. Hence the aims of the present study were 1) to establish a comparable and reliable translational model of mechanical rectal distension, 2) to compare the sensory response between species in terms of CEPs, and 3) to calculate the reproducibility of the method within and between days.
MATERIALS AND METHODS
Rat Experiment
Animals.
Twelve female Sprague-Dawley rats (Harlan Laboratories, Venray, The Netherlands), weighing 250–300 g, were used. The rats were acclimatized to the animal facility for at least 1 wk. Rats were housed in groups of five in an enriched environment with free access to food (standard pellets, R3, Lactamin, Kimstad, Sweden) and water on a 12:12-h light-dark cycle. The estrous stage of the rats was not accounted for in the present study. All experiments were approved by the local animal ethics review committee in Göteborg, Sweden (403-2008) and conducted at AstraZeneca, Mölndal, Sweden.
Surgery.
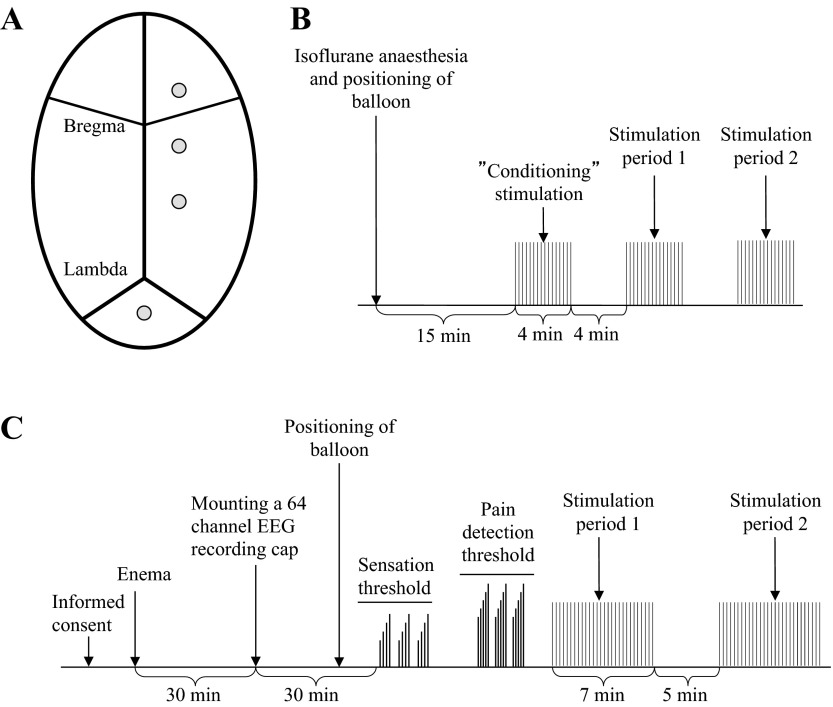
Prior to surgery, rats were administered antibiotics (1 ml/kg) (Bactrim, Oral 40/8 mg/ml, Roche, Basel, Switzerland) and analgesic (10 mg/kg) (Romefen, Merial Norden, Skovlunde, Denmark). Rats were anesthetized with isoflurane (2–3 vol%) (Forene, Abbott Scandinavia, Solna, Sweden) and kept on a heating pad during surgery to maintain body temperature. Surgical procedures consisted of implantation of skull electrodes and positioning of an abdominal connector. A midline scalp incision was made and the abdominal cavity was exposed by a 5-cm midline incision. From the abdominal cavity, Teflon-coated stainless steel cables (Ø = 0.3 mm; Cooner Wire, Chatsworth, CA) were passed through the abdominal musculature and tunneled subcutaneously across the thorax to the incision on top of the head. Two centimeters lateral to the abdominal incision a small plastic connector (AstraZeneca) was exteriorized and sutured to the abdominal wall to allow future access to the electrode cables. The abdominal incision was closed and the rats were then placed in a stereotaxic frame. A few drops of lidocaine (Xylocaine iv, 20 mg/ml, AstraZeneca, Södertälje, Sweden) were applied to the scalp incision and connective tissue was scraped away from the skull surface. After exposure of the skull, a small hole (Ø0.9 mm) was drilled on each of the electrode positions and cables were secured by titanium electrodes (Ø0.9 mm, Wennbergs finmek, Gunnilse, Sweden) into these positions. Three electrodes for monopolar recordings were placed on the right side of the skull (Fig. 1A); the positions were selected based on experience from a previous study (23). Briefly, three electrodes were placed 1.5 mm lateral to the sagittal line. The most anterior electrode was placed 1.5 mm anterior to bregma and the following two electrodes in a rostrocaudal direction, separated by 3 mm (Fig. 1A). The reference electrode was positioned 2 mm caudal to lambda.
Fig. 1.
A: positioning of recording electrodes on the rat skull. B: schematic drawing of the experimental protocol used in the rat study. C: schematic drawing of the experimental protocol used in the human experiment.
Electrodes were placed avoiding penetration of the underlying dura mater. Dental cement (Revolution Formula 2, Kerr, Orange, CA) was applied to fasten the screws and cables to the surface of the skull.
Subcutaneous injections of glucose (Rehydrex 25 mg/ml, Fresenius Kabi, Bad Homburg, Germany) and buprenorphine (0.04 mg/kg) (Temgesic 0.3 mg/ml, RB Pharmaceuticals, Berkshire, UK) were given postoperatively. The animals recovered from surgery in a quiet and dim postoperative room for 24 h. They were treated with antibiotics (1 ml/kg) (Bactrim, Oral 40/8 mg/ml, Roche) and analgesics (10 mg/kg) (Romefen, Merial Norden, Skovlunde, Denmark) 3 days postoperatively. To avoid postsurgical complications, the rats were housed individually in cages for up to 14 days following the surgical procedure. The rats were then group housed and were included in studies for up to 6 mo.
Stimulation device.
Rectal distensions were delivered by using a 3-cm-long polyethylene balloon (noncompliant material) with a maximal diameter of 10 mm (made in-house) secured to a connecting catheter (30 cm length, 2 mm inner diameter). The connecting catheter was fixed to the tail with adhesive tape and connected to a customized barostat (AstraZeneca) that was used to manage air inflation. Customized computer software (PharmLab on-line 6.0, AstraZeneca) running on a standard computer was used to control the barostat and to record data. The intraballoon pressure was measured in vivo by a miniature pressure transducer (Micro-tip transducer, Millar Instruments, Houston, TX). The pressure could be increased from 0 to 90% of maximal pressure within less than 20 ms and returned to 10% of baseline pressure within less than 10 ms.
Electroencephalographic recordings and analysis.
The EEG was recorded by use of an in-house-built amplifier (gain 10,000, bandwidth 0.3 Hz–1 kHz) at a sampling frequency of 2,000 Hz. The recordings were obtained in a room with dimmed lights, and all unnecessary electrical equipment was turned off to avoid 50-Hz contamination of the signals. The EEG signals were filtered with a 0.3- to 200-Hz band-pass filter. The signals were stored for further analysis by using in-house developed software for EEG analysis (PharmLab 6.0, AstraZeneca). The EEG signal recorded in the interval 0–400 ms after stimulation was used for analysis of the CEPs. CEPs were generated from averaging EEG signals recorded in each session, and offline analysis of the averaged CEPs was performed with customized software.
Protocol.
A schematic presentation of the protocol is provided in Table 1 and Fig. 1B.
Table 1.
Schematic comparison of the rat and human models
| Rat Model | Human Model | |
|---|---|---|
| EEG electrodes | Permanently implanted in the skull; 3 recording electrodes | Mounted externally; 62 recording electrodes |
| Balloon | ||
| length | 3 cm | 3 cm |
| max. circumference | 1.6 cm | 17.5 cm |
| position | 3 cm cranial to anal verge | 10 cm cranial to anal verge |
| Stimuli | ||
| duration | 100 ms | 150 ms |
| intensity | 80 mmHg | Individualized at VAS 5, max. pressure 30 psi |
| interstimulus interval | 4 ± 1 s | 12 ± 4 s |
| number of stimuli | 60 | 30 |
| Response | EEG/CEP | EEG/CEP + VAS |
VAS, visual analog scale; CEP, cerebral evoked potential; max., maximum.
To reduce motion artifacts due to restraint stress, the rats were habituated to sit in a Bollmann cage (Plexiglas tubes, length 18 cm, diameter 6 cm; AstraZeneca), 30 min per day for 3 consecutive days prior to experiments. During light isoflurane anesthesia (2.0% vol/vol) (Forene, Abbott Scandinavia, Solane, Sweden), the balloon was placed in rectum, 3 cm cranial to the anal verge. Rats were allowed to recover from sedation in the Bollmann cages for at least 15 min before the start of experiments.
Rectal distension was performed by rapid balloon distension, consisting of a phasic 100-ms-long pressure pulse at 80 mmHg with a random interstimulus interval of 4 ± 1 s, corresponding to a mean frequency of 0.25 Hz. Three periods of stimulation were recorded for each experiment. Each period was separated by 4 min of rest (Fig. 1B). The first period of stimulation served to habituate the rat through familiarization of the nature of the stimulation and environment, with the aim of increasing the reproducibility of responses during the next two periods (15). To evaluate reproducibility between days, each rat participated two times separated by minimum 1 wk (mean: 24 days; range 7–80 days).
Because the balloon material was noncompliant, the force applied to the rectal wall can be assumed to be equal to the stimulation pressure of 80 mmHg. During in vitro test, in which the balloon was distended outside the animal, there was no increase in balloon pressure during distension, until the balloon was distended to its fully capacity.
Human Experiment
Subjects.
Eighteen healthy volunteers (12 men and 6 women, mean age 34 yr, range 21–56 yr, SD 13.8) were recruited from hospital and university staff. All were healthy adults with no history of medical, gastrointestinal, or neurological disorders. They were not taking any medication on a regular basis, except contraceptive pills. Female subjects were studied between day 6 and 19 of the menstrual cycle (1, 25, 30, 49). All subjects gave informed consent prior to the experiment, carried out at the Department of Gastroenterology and Hepatology, Aalborg Hospital, Denmark. The experiment was authorized by the local Ethical Committee (N-20090008).
Sensory assessment.
INDIVIDUAL ANXIETY ASSESSMENT.
All subjects completed the Spielberger State-Trait Anxiety Inventory (STAI) questionnaire (22) to assess state (anxiety level on the day of the experiment) and trait (general anxiety levels over the past few weeks/months) anxiety (STAI range, 20–80; higher scores represent higher anxiety). The questionnaire (which is not a screening or clinical tool and measures a normal range of anxiety) was administered immediately prior to the experiment. These data were collected to assess anxiety between sessions because it is well known that anxiety levels can affect pain reports [thresholds and visual analog scale (VAS) responses], which may therefore also influence the CEPs (45).
VISUAL ANALOG SCALE.
All volunteers were instructed on the use of a modified VAS (0–10), where 0 = no perception, 1 = vague perception of mild sensation (sensation threshold), 2 = definite perception of mild sensation, 3 = vague perception of moderate sensation, 4 = definite perception of moderate sensation, 5 = first sensation of pain (pain detection threshold), 6 = mild pain, 7 = moderate pain, 8 = pain of medium intensity, 9 = intense pain, and 10 = unbearable pain. The scale has been described in detail previously (11) and has been shown to be robust and valid in assessing rectal sensation in experimental and clinical studies (8, 10, 44).
Stimulation device.
A 33-cm Ryles tube CH/FG16 (5.3 mm diameter) (RT2016/L, Pennine Healthcare, Derby, UK) with a 3-cm nitrile rubber balloon (compliant material) secured with suture to one end was used to produce mechanical rectal distensions. The catheter was attached to a specially designed inflator device (Medical Physics Department, Hope Hospital, Manchester, UK), with a maximum stimulation pressure of 30 psi, resulting in a maximum balloon volume of 83 ml and a corresponding circumference of 17.5 cm. In this setup, it was not possible to measure the actual pressure inside the balloon; therefore the measured pressure was the pressure in the air tank inside the pump. A digitalized trigger signal was used to synchronize the EEG recording and balloon distension. However, the inflation device produced a time delay of 35 ms from trigger signal to onset of balloon inflation (due to the length of tubing between the inflation device and balloon) for which all latencies were corrected. Stimulation intensity was titrated at a pressure corresponding to pain detection threshold (the point at which volunteers first reported pain, point 5 on the VAS) in each individual volunteer.
Electroencephalographic recordings and analysis.
The multichannel EEG was recorded from 62 electrodes by using an amplifier (SynampII, Neuroscan, El Paso, TX) and a standard EEG cap (Quick-Cap International, Neuroscan) mounted according to the extended international 10–20 system (26). The impedance of the electrodes was on average kept below 5 kΩ. EEG signals were recorded with a sampling frequency of 1,000 Hz. The recordings were obtained in a dimmed room, and all unnecessary electrical equipment was turned off to avoid 50-Hz contamination of the signals. Subjects were instructed to lie relaxed, eyes open, and were told to fixate their gaze on a remote object. Evoked potentials were generated from averaging EEG signals recorded in each session.
Offline analysis of the averaged CEPs was done using commercial software (Neuroscan version 4.3.1, Neuroscan). The procedure included the following preprocessing steps: 1) band-stop 49–51 Hz (notch filtering), 2) band-pass filtering (1–70 Hz), 3) epoching −50 to 700 ms poststimulus, 4) linear detrending, 5) baseline correction, 6) manual rejection of artifact sweeps, 7) rereferencing to linked ear, and 8) calculating average of accepted sweeps.
Protocol.
A schematic presentation of the protocol is provided in Table 1 and Fig. 1C.
Each volunteer participated on two occasions separated by at least 4 days (mean: 9 days; range 4–21 days); during each occasion the same protocol was repeated. Forty minutes prior to stimulation a bisacodyl enema (Toilax, Orion Pharma, Nivå, Denmark) was administered. Following this, the EEG cap was mounted and subjects were asked to lie on their left side and the lubricated stimulation probe was positioned until the distal end of the balloon was 10 cm cranial to the anal verge.
Detection of sensation threshold was achieved by slowly increasing the stimulation pressure until the subject reported the first sensation of balloon inflation. This procedure was repeated three times, and the mean value was calculated. The pain detection threshold was determined by continuing to slowly increase the pressure until the subject reported a feeling of pain for the first time. This individualized pressure at pain detection threshold was used throughout the subsequent experiment. The detection of sensory and pain detection threshold also served as preconditioning, thereby avoiding problems associated with the viscoelastic properties of the gut and enabling training of the subjects to help ensure reproducible responses on subsequent stimulations (15, 44). For safety reasons, the maximum stimulation pressure was limited to 30 psi. If subjects failed to reach pain detection threshold at 30 psi, the subsequent stimulations were delivered at this maximum intensity. Because the balloon was made of nitrile rubber, which is a compliant material, the stimulation pressure inside the balloon (which could not be measured) is partly related to the balloon compliance and partly related to the properties of the rectal wall. Because of this, is not possible to deduct the force applied to the gut wall based on the measured pressure.
To test for reproducibility within the same day, two recordings of EEG were performed, separated by 5 min. For each recording, 30 identical phasic rectal stimuli were delivered, each lasting 150 ms with a random interstimulus interval of 12 ± 4 s, equivalent to a mean stimulation frequency of 0.08 Hz (16). The subject assessed the sensation on the VAS after each stimulus. Following the first recording, there was 5 min of rest (no stimulation) before the second period of stimulation commenced.
Data Analysis
Cerebral evoked potentials.
In both rats and humans, the morphology of the CEP waveforms consisted of a triphasic response characterized as prominent negative (N) and positive (P) peaks numbered in order of occurrence, i.e., N1, P1, etc. The latency (ms) of the cortical responses was measured at the peak of the distinct negative and positive peaks. The cortical amplitude (μV) was measured for the first peak (P1) relative to baseline and for the following peak-to-peak (P1–N1 and N1–P2).
Rats.
Maximal amplitudes were recorded at the electrode 1.5 mm posterior of bregma and 1.5 mm lateral of the midline, and hence recordings from this electrode were used for further analysis. EEG signals were analyzed in the interval 0–300 ms after stimulation. Each CEP recorded was manually inspected and the first positive peak was identified and labeled P1. The following distinct negative and positive peaks were identified and labeled N1, P2, etc. The amplitude and latency was determined for each peak. Reproducibility of amplitude, latency, and spectral analysis within and between days were analyzed by comparing responses from the two stimulation periods on each day.
Humans.
Recordings from the vertex electrode (Cz) were used for analysis since brain activation following lower gut stimulation previously has been reported in centrofrontal regions (13, 47). EEG signals were analyzed in the interval 0–500 ms after stimulation and the different peaks were identified as described for the rats. Reproducibility of amplitude, latency, and spectral analysis within and between days were analyzed by comparing responses from the two stimulation periods on each day.
Spectral analysis of cerebral evoked potentials.
EEG power distributions for each recording file were estimated by a continuous wavelet transform, designed with a complex Morlet wavelet to compute the time-frequency coefficients with a resolution of 0.5 Hz (14). The Morlet wavelet is defined by two design parameters, which for this study were set to have a bandwidth parameter of 10 Hz and center frequency of 1 Hz. These parameters were chosen to obtain a favorable frequency resolution of the EEG (48). The coefficients were squared to obtain the power coefficients, followed by integration over time and scales to calculate the power distribution in the frequency bands: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–32 Hz), and gamma (32–80 Hz).
Statistics
All data are represented as means ± SD unless stated otherwise. To test the within- and between-session differences, a two-way ANOVA was used for statistical analysis (SigmaStat 3.0, SPSS, Chicago, IL). P values below 0.05 were considered statically significant. Intraclass correlation coefficients (ICC) were calculated to evaluate the intraindividual variance, representing the reproducibility. ICC values describe the variation within the individual subject in response to repeated stimulations compared with the variation between all subjects. The ICC was calculated as
where σwithin is the variation within individuals and σtotal is the sum of variance within individuals and between the repetitions of the test (52). ICC values ranges from 0 to 1, with values closest to 1 indicating the highest reproducibility. Previously the acceptable level of ICC has been set to ≥0.6 (2, 5, 6, 52). Correlations between measurements were calculated as Spearman correlations.
RESULTS
Rat Experiments
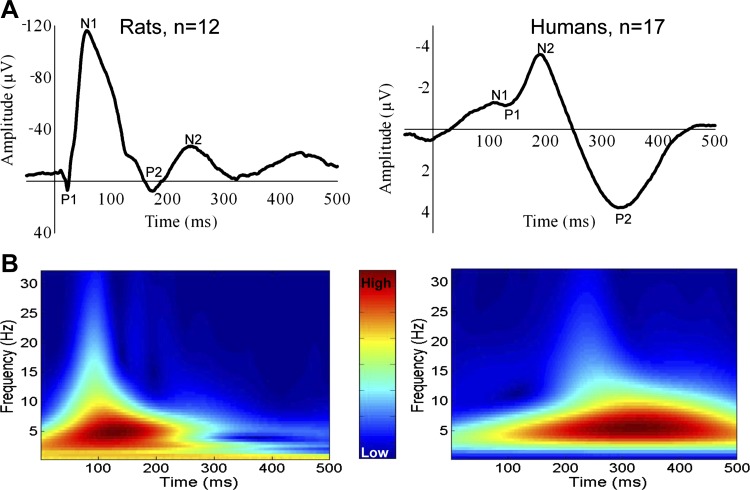
Evoked potentials to rectal rapid balloon distension were recorded successfully in all 12 rats, and no adverse reactions to stimulation were observed. The CEPs had a common morphology consisting of at least three different, partly overlapping components, labeled P1, N1, and P2 (Fig. 2A). A late negative component (N2) was present in 22 of 48 (46%) of recorded CEPs and could be difficult to locate unambiguously. Therefore, N2 was excluded in further analysis. Data are presented in Table 2.
Fig. 2.
A: grand mean of cerebral evoked potentials (CEPs) from all subjects. The different components on the CEPs are marked on the graphs. The mean CEPs from the human subjects have been aligned according to N2 to adjust for latency jitter. B: time-frequency plots of grand mean CEPs for rats and humans. The main power for rats was within the theta band (4–8 Hz), whereas the main power for humans was in the delta band (0.5–4 Hz).
Table 2.
Descriptive analysis of CEPs recorded from rats
| Latency, ms |
Amplitude, μV |
Distribution of Frequency Content, % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation Period | P1 | N1 | P2 | P1 | P1–N1 | N1–P2 | Delta | Theta | Alpha | Beta | Gamma |
| Day 1 | |||||||||||
| 1 | 18.8 (5.2) | 60.2 (16.3) | 159.6 (24.6) | 18.4 (17.3) | 145.1 (48.5) | 150.5 (50.6) | 20.9 (10.0) | 45.7 (12.8) | 15.3 (6.6) | 15.8 (5.9) | 2.4 (1.0) |
| 2 | 20.0 (4.8) | 65.2 (16.6) | 163.7 (10.7) | 14.7 (22.5) | 152.7 (47.1) | 153.5 (54.0) | 25.3 (10.4) | 45.6 (14.5) | 13.0 (5.9) | 14.4 (7.3) | 1.7 (0.7) |
| Day 2 | |||||||||||
| 1 | 18.3 (3.8) | 60.4 (16.6) | 168.2 (28.8) | 16.0 (6.6) | 145.7 (46.8) | 156.7 (55.4) | 26.2 (10.4) | 43.9 (14.9) | 13.4 (9.4) | 13.3 (8.0) | 3.2 (2.1) |
| 2 | 18.0 (4.2) | 57.3 (11.4) | 171.1 (17.0) | 14.5 (9.4) | 147.9 (48.2) | 155.2 (50.2) | 30.4 (12.8) | 44.2 (15.6) | 10.9 (5.9) | 12.1 (10.0) | 2.4 (1.7) |
All values are presented as means (SD); N =12 rats. The CEPs are described in relation to latency, amplitude, and distribution of frequency content.
The spectral analysis of the CEPs showed that EEG power was contained mainly in the delta and theta bands whereas a smaller part was distributed to the alpha, beta, and gamma bands (Table 2 and Fig. 2B).
Reproducibility within day.
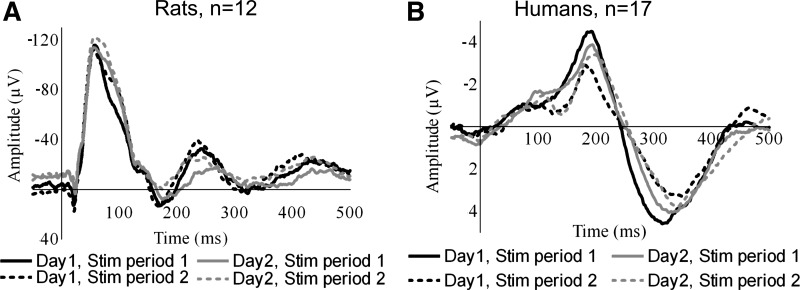
No statistically significant differences between stimulation periods 1 and 2 were shown for latencies (F = 0.4; P = 0.5), amplitudes (F = 0.02; P = 0.9), or spectral analysis (F < 0.001; P ≥ 0.9). Figure 3 displays reproducibility of the grand mean within and between days. Peak-to-peak amplitude P1–N1 and N1–P2 were the most reproducible parameter displaying very high ICC values on both days (see Table 3). Power distribution (Table 3) showed reproducibility in the delta, theta, and alpha bands. The beta and gamma bands were less consistent, only being reproducible on the second day.
Fig. 3.
Reproducibility of CEPs. Stim, stimulation.
Table 3.
Reproducibility of cerebral evoked potentials from rats
| Latency |
Amplitude |
Frequency Distribution |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | N1 | P2 | P1 | P1–N1 | N1–P2 | Delta | Theta | Alpha | Beta | Gamma | |
| Within-day reproducibility | |||||||||||
| Day 1 | 0.98 | 0.91 | 0.67 | 0.91 | 0.99 | 0.99 | 0.96 | 0.86 | 0.90 | 0.59 | 0.53 |
| Day 2 | 0.98 | 0.82 | 0.93 | 0.51 | 0.99 | 0.98 | 0.96 | 0.95 | 0.85 | 0.96 | 0.86 |
| Between-day reproducibility | |||||||||||
| Stimulation period 1 | 0.61 | 0.69 | 0.42 | 0.66 | 0.98 | 0.98 | 0.39 | 0.50 | 0.78 | 0.74 | 0.60 |
| Stimulation period 2 | 0.57 | 0.51 | 0.50 | 0.67 | 0.93 | 0.90 | 0.39 | 0.40 | 0.46 | 0.42 | 0.56 |
N =12 rats. The reproducibility is evaluated by intraclass correlation coefficients within and between days. Values above 0.6 indicate acceptable level of reproducibility.
Reproducibility between days.
No statistical significant differences between days 1 and 2 were shown for latencies (F = 0.1; P = 0.7), amplitudes (F < 0.001; P ≥ 0.9), or distribution of EEG power between bands (F < 0.001; P = 0.5). Peak-to-peak amplitude P1–N1 and N1–P2 was the most reproducible parameter, displaying high ICC values in both stimulation periods (see Table 3). In general the reproducibility of EEG power distribution between days was poor.
Human Experiments
Evoked potentials to rapid rectal balloon distension were recorded successfully in all 18 subjects; however, one subject had a fracture of the hand between the two visits and was excluded because of pain from the fracture site.
Sensory perception.
Five of the 17 subjects failed to reach the pain detection threshold at maximum balloon pressure, two of these on both days. The average rating of the 30 stimuli was not significantly different between subjects that reached pain threshold and those that failed (3.6 vs. 3.9, P value = 0.53). Furthermore, no statistically significant differences were apparent between the two groups with respect to amplitude and latency of the CEPs (all P values <0.05). Since there were no significant differences between the two groups, subjects who failed in reaching the pain threshold were included in the analysis. Stimulation pressure between days was reproducible [22.4 psi (SD 7.8) vs. 20.6 psi (SD 9.1); ICC = 0.98]. VAS responses were reproducible within day 1 [3.88 (SD 1.0) vs. 3.80 (SD 1.2); ICC = 0.99] and day 2 [3.75 (SD 0.9) vs. 3.73 (SD 0.9); ICC = 0.99] and between days in stimulation period 1 [3.88 (SD 1.0) vs. 3.75 (SD 0.9); ICC = 0.96] and stimulation period 2 [3.80 (SD 1.2) vs. 3.73 (SD 0.9); ICC = 0.97].
Anxiety assessment.
All subjects had low trait anxiety with a mean score of 24.2 (SD 9.9; range 20–31). The state anxiety score was also low and was reproducible between days [23.8 (SD 3.4) vs. 21.8 (SD 2.2); ICC = 0.64]. No significant correlation between anxiety score and stimulation pressure was seen [day 1: r = −0.34 (P = 0.2); day 2: r = −0.47 (P = 0.07)].
Cerebral evoked potentials.
In all 17 subjects CEPs were recorded successfully, presenting similar triphasic morphology consisting of a common P1–N2–P2 wave form (Fig. 2A). An early negative component (N1) was present in 41 of the 68 (60%) recorded CEPs, but no subject had N1 present in all four recordings. Since N1 was hard to locate unambiguously, it was excluded in further analysis. The first analyzed component, P1, was also relatively small; however, it could be identified in 54 of 68 (79%) of the recordings. The amplitudes and latencies for all peaks are given in Table 4. There were no correlations between the stimulation pressure and the amplitudes of the CEPs [day 1: P1: r = 0.11 (P = 0.53); P1–N2: r = 0.19 (P = 0.29); N2–P2: r = −0.03 (P = 0.88); day 2: P1: r = −0.19 (P = 0.28); P1–N2: r = 0.17 (P = 0.34); N2–P2: r = 0.31 (P = 0.07)]. The spectral analysis of the CEPs showed that EEG power were contained mainly in the delta (47.2%, SD 15.7) and theta (33.2%, SD 13.2) bands whereas a smaller part were distributed to the alpha, beta, and gamma bands (Table 4).
Table 4.
Descriptive analysis of CEPs recorded from humans
| Latency, ms |
Amplitude, μV |
Distribution of Frequency Content, % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation period | P1 | N2 | P2 | P1 | P1–N2 | N2–P2 | Delta | Theta | Alpha | Beta | Gamma |
| Day 1 | |||||||||||
| 1 | 114.6 (30.1) | 185.8 (36.7) | 312.8 (42.5) | 0.33 (2.1) | 6.9 (5.8) | 15.5 (6.6) | 47.6 (12.8) | 34.9 (12.4) | 11.7 (7.0) | 5.7 (0.3) | 0.2 (0.1) |
| 2 | 117.2 (38.3) | 178.3 (45.8) | 318.4 (31.3) | 0.85 (2.5) | 7.1 (4.1) | 13.5 (5.4) | 40.9 (16.5) | 34.1 (12.0) | 17.5 (14.8) | 7.3 (4.9) | 0.3 (0.2) |
| Day 2 | |||||||||||
| 1 | 116.4 (35.0) | 192.5 (37.1) | 320.0 (33.8) | −0.2 (2.5) | 7.5 (3.7) | 16.1 (5.4) | 49.9 (16.9) | 32.2 (13.6) | 11.1 (12.0) | 6.5 (4.9) | 0.2 (0.1) |
| 2 | 126.1 (40.0) | 190.2 (39.6) | 324.7 (44.1) | 0.31 (1.6) | 6.6 (2.9) | 13.9 (4.1) | 50.5 (15.7) | 31.4 (14.8) | 10.0 (6.2) | 7.9 (4.5) | 0.3 (0.2) |
All values are presented as means (SD); N =17 subjects. The CEPs are described in relation to latency, amplitude, and distribution of frequency content.
Reproducibility within day.
No statistical significant differences between stimulation periods 1 and 2 were shown for latencies (F = 0.2; P = 0.6), amplitudes (F = 1.2; P = 0.3), or spectral analysis (F < 0.001; P ≥ 0.9). Latencies and amplitudes were reproducible showing high ICC values on day 1 (all ICC values ≥ 0.84) and day 2 (all ICC values ≥ 0.87). Data are listed in Table 5. The reproducibility of the power distribution (Table 5) showed that the content in the delta, theta, and alpha bands were reproducible (all ICC values >0.60). The beta and gamma bands were less consistent, displaying unacceptable levels of reproducibility between days.
Table 5.
Reproducibility of cerebral evoked potentials from humans
| Latency |
Amplitude |
Frequency Distribution |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | N2 | P2 | P1 | P1–N2 | N2–P2 | Delta | Theta | Alpha | Beta | Gamma | |
| Within-day reproducibility | |||||||||||
| Day 1 | 0.98 | 0.98 | 0.93 | 0.90 | 0.93 | 0.84 | 0.71 | 0.61 | 0.80 | 0.78 | 0.68 |
| Day 2 | 0.95 | 0.98 | 0.96 | 0.88 | 0.87 | 0.89 | 0.95 | 0.92 | 0.81 | 0.77 | 0.53 |
| Between-day reproducibility | |||||||||||
| Stimulation period 1 | 0.91 | 0.87 | 0.91 | 0.83 | 0.83 | 0.70 | 0.90 | 0.91 | 0.90 | 0.73 | 0.54 |
| Stimulation period 2 | 0.82 | 0.91 | 0.89 | 0.95 | 0.87 | 0.78 | 0.79 | 0.64 | 0.60 | 0.56 | 0.52 |
N =17 subjects. The reproducibility is evaluated by intraclass correlation coefficients within and between days. Values above 0.6 indicate acceptable level of reproducibility.
Reproducibility between days.
No statistical significant differences between days 1 and 2 were shown for latencies (F = 2.0; P = 0.2), amplitudes (F < 0.001; P ≥ 0.9) or spectral analysis (F < 0.001; P ≥ 0.95). Latencies and amplitudes were reproducible between days, showing high ICC values in stimulation period 1 (ICC ≥ 0.70) and stimulation period 2 (ICC ≥ 0.78). Figure 3 displays reproducibility of the grand mean within and between days. Spectral analysis showed good reproducibility in delta, theta, and alpha bands (Table 5). However, beta and gamma bands were less consistent.
DISCUSSION
A unique visceral translational platform was established to reliably assess CEPs in terms of latencies, amplitude, and spectral analysis to rapid balloon distension in rats and humans.
In rats, the model was shown to be accurate, responsive to stimulation, sensitive to peripheral blockade, and arguably an improvement on electrically elicited CEPs since the stimulus is more physiological. The present study proves that the human model is robust, although further studies are needed to probe response to varying stimulation intensities and sensitivity to therapeutic intervention. Despite this, the translational neurophysiological approach enables the possibility to study basic visceral sensitivity. This could improve the process of developing new drugs targeting visceral pain, since the use of a common model in both animals and humans will promote the successful translation of results between species.
Neurophysiological Considerations
Sensation from the distal colon and rectum is conveyed to the central nervous system through two distinct anatomical pathways: the lumbar splanchnic nerves, terminating in the thoracolumbar spinal cord, and the pelvic nerves, terminating in the lumbosacral spinal cord. Recent results from animal experiments indicate that acute noxious distension of the colorectum is transmitted predominantly through the pelvic nerves (28). The majority of the colorectal distension-responsive afferents are unmyelinated C fibers, with only ∼20% myelinated Aδ-fibers (50, 53). Low-threshold fibers displaying increasing firing in response to increasing stimulation (e.g., from physiological to supraphysiological pressures) constitute the major component of the responsive afferents. The firing rate of high-threshold fibers (responsive to stimuli above 28 mmHg) is lower than for the low-threshold fibers, which are also more frequently represented; hence, the total number of impulses in response to a given stimulus pressure mainly originates from low-threshold fibers (50).
The CEPs recorded are not specific to noxious stimulus or noxious sensation, but rather related to the stimulus or the evoked sensation, regardless of it being noxious or nonnoxious. Hultin et al. (23) showed that CEPs in rats can be reliably elicited by rapid balloon distension of both nonnoxious (pressure 20 mmHg) and noxious (pressure 80 mmHg) stimulus intensities and that the amplitude of the CEP was related to the stimulus intensities. Human experiments with mechanical colorectal distension and CEPs have also shown that nonnoxious stimulus intensities can elicit CEPs (7, 16, 18, 31).
Latencies in the present study were less than 400 ms. Because the rectum is mainly innervated by Aδ- and C fibers (with conduction velocities 5–25 and <2.5 m/s), the CEPs to rectal distension are expected to occur with latencies longer than 10 ms in rats and 40 ms in humans (34, 40). Furthermore, selective activation of C fiber afferents by laser CEPs in humans showed latencies above 800 ms (9, 54). Taken together, the results indicate that CEPs in this study primarily were mediated via low-threshold Aδ-fibers.
Methodological Considerations
Given that subjective pain assessment in the rats is not possible, the stimulation protocol was based on our collective experience from previous experiments. Hence, a noxious stimulation pressure of 80 mmHg was used (4, 31, 39, 42). In humans, subjective assessment of the evoked sensation made it possible to obtain the “true” sensation and individualize the stimulus intensity corresponding to pain detection threshold. However, one difficulty with using this stimulation level is that rectal mechanical distension does not always produce a painful sensation and instead induces a strong urge to defecate before pain levels can be reached (15, 16). This was consistent with findings in the present study, as five subjects did not reach pain detection threshold at the maximum inflation volume.
In this study, a sufficient synchronization between pump onset and afferent nerve discharge made it possible to identify the individual triphasic morphology. However, the lag time between trigger signal and the stimulation pressure were different between subjects, since stimulation pressure was individualized. This phenomenon induces a “latency jitter,” which can mask the earliest negative components of the CEPs, and hence it can be challenging to locate the component consistently. However, in contrast to previous studies, in which CEPs to rapid balloon distension were recorded less consistently than CEPs to electrical stimulation, the present study demonstrates high reproducibility of amplitudes and latencies within and between days, being comparable to those elicited electrically (3, 16, 18). In the present study, both rats and humans had reproducible CEPs with triphasic morphology, revealing peak-to-peak amplitude as the most reproducible parameter within and between days, seen across both species. Generally, rats showed a lower reproducibility of latencies between days, which could be caused by variation in the exact probe position, amount of fecal pellets in colon, or differences in anxiety level. Owing to epidural position of recording electrodes, the amplitude of the rat CEPs was ∼10-fold, larger than amplitudes in the human CEPs. However, even though the recorded signal in humans was attenuated by passage of meninges, skull, and skin, the potentials were robust and reliably assessed.
Spectral Analysis
The spectral analyses of the CEPs showed that the EEG power was distributed mainly in the delta and theta bands. The main power in rats was contained in the theta band, whereas humans had the predominant power in the delta band. The spectral analysis reflects directly the differences in CEP morphology between rats and humans. The first negative component (N1) in the rat CEPs is narrower, which is reflected in theta power, and the human components are broader, containing more delta power. This finding could reflect differences in stimulation characteristic. However, it could also reflect differences in cortical activation.
Translational Approach
Recently, a novel rat model combining CEPs and colorectal distension has been established in conscious rats. The model was tested with different stimulation intensities and showed association between stimulation pressure and amplitude of the CEPs (23), indicating that brain activity was responsive to the peripheral stimulus. Furthermore, the model was refined by rectal administration of lidocaine, which diminished the amplitude of CEPs, indicating that the model was sensitive to peripheral blockade of sensory receptors. Regrettably, the model was not combined with simultaneous recording of the visceromotor response. If this had been established, an association between the widely used visceromotor reflex and CEPs could have been investigated. This is highly recommended in future designs.
Inverse modeling studies of human visceral CEPs have predominantly shown activation of the insula cortex, secondary somatosensory cortex, and cingulate cortex (3, 10, 43). It is widely accepted that the prominent centrally distributed N2–P2 component of human visceral CEPs represents the endogenous pain response mainly through cingulate activation (21). Because of anatomical brain differences, comparison of CEPs and the underlying brain activity in rats and humans should be interpreted cautiously. However, functional magnetic resonance imaging studies of both rat and human brains have shown activation of the same brain structures during colorectal distension (36, 56). The same structures are likely activated in the present study, and hence the recorded CEPs may reflect comparable activation of the neuroaxis in rats and humans.
In conclusion, the present study has established an experimental model of visceral sensitivity, which can be applied in both rats and humans. This could prove an important step on the path to establishing experimental models with higher translational value. However, further studies are needed to illuminate the strengths and drawbacks of the models since there are still challenges regarding the translational approach to overcome. Continuing to develop improved complementary human-animal models could facilitate the process of developing new drugs targeting visceral pain, since the use of a common model in both animals and humans will promote the successful translation of results between species.
GRANTS
The research at Aalborg Hospital leading to these results has received funding from the Danish Agency for Science, Technology and Innovation: Det Strategiske Forskningsråd grant no. 10-092786 and Det Obelske Familie Fond. Q. Aziz was funded by the Medical Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.D.N., C.B., and L.H. conception and design of research; T.D.N., C.B., and C.G. performed experiments; T.D.N. and C.G. analyzed data; T.D.N. and C.B. interpreted results of experiments; T.D.N. prepared figures; T.D.N., C.B., and C.G. drafted manuscript; T.D.N., S.J.C., L.H., Q.A., J.L., and A.M.D. edited and revised manuscript; T.D.N., C.B., C.G., S.J.C., L.H., Q.A., J.L., and A.M.D. approved final version of manuscript.
REFERENCES
- 1. Arendt-Nielsen L, Bajaj P, Drewes AM. Visceral pain: gender differences in response to experimental and clinical pain. Eur J Pain 8: 465–472, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Brock C, Nissen TD, Gravesen FH, Frokjaer JB, Omar H, Gale J, Gregersen H, Svendsen O, Drewes AM. Multimodal sensory testing of the rectum and rectosigmoid: development and reproducibility of a new method. Neurogastroenterol Motil 20: 908–918, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Brock C, Olesen SS, Valeriani M, Arendt-Nielsen L, Drewes AM. Brain activity in rectosigmoid pain: unravelling conditioning pain modulatory pathways. Clin Neurophysiol 123: 829–837, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Brusberg M, Ravnefjord A, Lindgreen M, Larsson H, Lindstrom E, Martinez V. Oral clonidine inhibits visceral pain-related viscerosomatic and cardiovascular responses to colorectal distension in rats. Eur J Pharmacol 591: 243–251, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bruton A, Conway JH, Holgate ST. Reliability: what is it, and how is it measured? Physiotherapy 86: 94–99, 2000 [Google Scholar]
- 6. Chinn S. Statistics in respiratory medicine. 2. Repeatability and method comparison. Thorax 46: 454–456, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collet L, Meunier P, Duclaux R, Chery-Croze S, Falipou P. Cerebral evoked potentials after endorectal mechanical stimulation in humans. Am J Physiol Gastrointest Liver Physiol 254: G477–G482, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Cremonini F, Houghton LA, Camilleri M, Ferber I, Fell C, Cox V, Castillo EJ, Alpers DH, Dewit OE, Gray E, Lea R, Zinsmeister AR, Whorwell PJ. Barostat testing of rectal sensation and compliance in humans: comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil 17: 810–820, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Domnick C, Hauck M, Casey KL, Engel AK, Lorenz J. C-fiber-related EEG-oscillations induced by laser radiant heat stimulation of capsaicin-treated skin. J Pain Res 2: 49–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drewes AM, Frokjaer JB, Larsen E, Reddy H, Arendt-Nielsen L, Gregersen H. Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm Bowel Dis 12: 294–303, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: a reappraisal of human studies. Scand J Gastroenterol 38: 1115–1130, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Drossman DA, Danilewitz M, Naesdal J, Hwang C, Adler J, Silberg DG. Randomized, double-blind, placebo-controlled trial of the 5-HT1A receptor antagonist AZD7371 tartrate monohydrate (robalzotan tartrate monohydrate) in patients with irritable bowel syndrome. Am J Gastroenterol 103: 2562–2569, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience 133: 533–542, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Durka PJ. From wavelets to adaptive approximations: time-frequency parametrization of EEG. Biomed Eng Online 2: 1, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol Gastrointest Liver Physiol 274: G584–G590, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Harris ML, Hobson AR, Hamdy S, Thompson DG, Akkermans LM, Aziz Q. Neurophysiological evaluation of healthy human anorectal sensation. Am J Physiol Gastrointest Liver Physiol 291: G950–G958, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hill R. NK1 (substance P) receptor antagonists–why are they not analgesic in humans? Trends Pharmacol Sci 21: 244–246, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hobday DI, Hobson A, Furlong PL, Thompson DG, Aziz Q. Comparison of cortical potentials evoked by mechanical and electrical stimulation of the rectum. Neurogastroenterol Motil 12: 547–554, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Hobday DI, Hobson AR, Sarkar S, Furlong PL, Thompson DG, Aziz Q. Cortical processing of human gut sensation: an evoked potential study. Am J Physiol Gastrointest Liver Physiol 283: G335–G339, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Hobson AR, Aziz Q, Furlong PL, Barlow JD, Bancewicz J, Thompson DG. Identification of the optimal parameters for recording cortical evoked potentials to human oesophageal electrical stimulation. Neurogastroenterol Motil 10: 421–430, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Hobson AR, Furlong PL, Worthen SF, Hillebrand A, Barnes GR, Singh KD, Aziz Q. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology 128: 610–619, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hodgues WF, Spielberger CD. An indicant of trait or state anxiety? J Consult Clin Psychol 33: 430–434, 1969 [DOI] [PubMed] [Google Scholar]
- 23. Hultin L, Nissen TD, Kakol-Palm D, Lindstrom E. Colorectal distension-evoked potentials in awake rats: a novel method for studies of visceral sensitivity. Neurogastroenterol Motil 24: 964–e466, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Iturrino J, Camilleri M, Busciglio I, Burton D, Zinsmeister AR. Effect of the α2δ ligand, pregabalin, on colonic sensory and motor functions in healthy adults. Am J Physiol Gastrointest Liver Physiol 301: G377–G384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson NA, Houghton LA, Whorwell PJ, Currer B. Does the menstrual cycle affect anorectal physiology? Dig Dis Sci 39: 2607–2611, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52: 3–6, 1999 [PubMed] [Google Scholar]
- 27. Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3: 711–715, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 107: 1686–1696, 1994 [DOI] [PubMed] [Google Scholar]
- 30. LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain 106: 253–261, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Lindstrom E, Brusberg M, Hughes PA, Martin CM, Brierley SM, Phillis BD, Martinsson R, Abrahamsson C, Larsson H, Martinez V, Blackshaw LA. Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain 137: 295–305, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Loening-Baucke V, Anderson RH, Yamada T, Zhu YX. Study of the afferent pathways from the rectum with a new distention control device. Neurology 45: 1510–1516, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Loening-Baucke V, Read NW, Yamada T. Cerebral evoked potentials after rectal stimulation. Electroencephalogr Clin Neurophysiol 80: 490–495, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Loening-Baucke V, Yamada T. Cerebral potentials evoked by rectal distention in humans. Electroencephalogr Clin Neurophysiol 88: 447–452, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 109: 40–52, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabate JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain 14: 142–148, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Murrell JC, Mitchinson SL, Johnstone AC, Johnson CB, Barnes GR. Is it possible to generate cerebral evoked potentials with a mechanical stimulus from the duodenum in rats? J Neurosci Methods 162: 215–221, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Murrell JC, Mitchinson SL, Waters D, Johnson CB. Comparative effect of thermal, mechanical, and electrical noxious stimuli on the electroencephalogram of the rat. Br J Anaesth 98: 366–371, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Ness TJ. Intravenous lidocaine inhibits visceral nociceptive reflexes and spinal neurons in the rat. Anesthesiology 92: 1685–1691, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain 41: 167–234, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13–L2 spinal cord of the rat. J Neurophysiol 60: 1419–1438, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Ness TJ, Randich A, Gebhart GF. Further behavioral evidence that colorectal distension is a ‘noxious’ visceral stimulus in rats. Neurosci Lett 131: 113–116, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Olesen SS, Frokjaer JB, Lelic D, Valeriani M, Drewes AM. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology 10: 742–751, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Petersen P, Gao C, Arendt-Nielsen L, Gregersen H, Drewes AM. Pain intensity and biomechanical responses during ramp-controlled distension of the human rectum. Dig Dis Sci 48: 1310–1316, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21: 9896–9903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ravnefjord A, Brusberg M, Larsson H, Lindstrom E, Martinez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol 155: 407–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rossel P, Arendt-Nielsen L, Niddam D, Chen AC, Drewes AM. Short latency cerebral response evoked by painful electrical stimulation applied to the human sigmoid colon and to the convergent referred somatic pain area. Exp Brain Res 151: 115–122, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Samar VJ, Bopardikar A, Rao R, Swartz K. Wavelet analysis of neuroelectric waveforms: a conceptual tutorial. Brain Lang 66: 7–60, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Sanoja R, Cervero F. Estrogen-dependent changes in visceral afferent sensitivity. Auton Neurosci 153: 84–89, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 71: 2046–2060, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Sikandar S, Dickenson AH. Pregabalin modulation of spinal and brainstem visceral nociceptive processing. Pain 152: 2312–2322, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Staahl C, Reddy H, Andersen SD, Arendt-Nielsen L, Drewes AM. Multi-modal and tissue-differentiated experimental pain assessment: reproducibility of a new concept for assessment of analgesics. Basic Clin Pharmacol Toxicol 98: 201–211, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 80: 2632–2644, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Tzabazis AZ, Klukinov M, Crottaz-Herbette S, Nemenov MI, Angst MS, Yeomans DC. Selective nociceptor activation in volunteers by infrared diode laser. Mol Pain 7: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Viramontes BE, Malcolm A, Camilleri M, Szarka LA, McKinzie S, Burton DD, Zinsmeister AR. Effects of an α2-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol Gastrointest Liver Physiol 281: G1468–G1476, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Wang Z, Bradesi S, Maarek JI, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain 138: 233–243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]