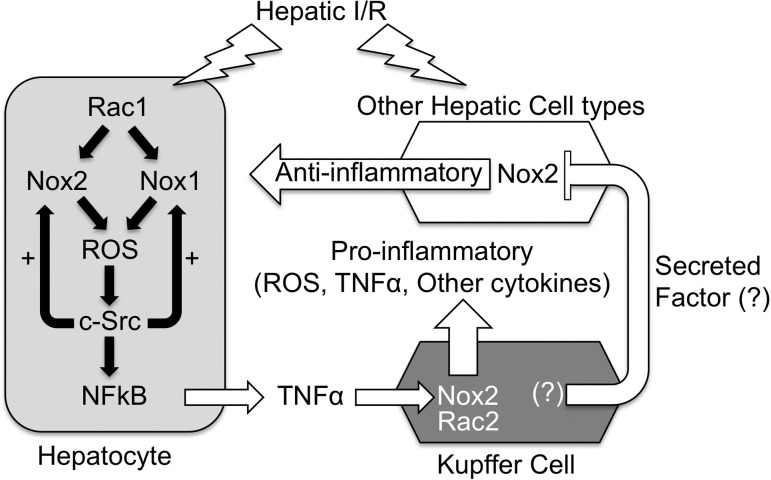
Fig. 6.
Working model for the NOX dependence of TNF-α production by the liver following I/R injury. We propose that the hepatocyte is one of the first cell types to respond to I/R injury by secreting low levels of NOX1/2-dependent TNF-α, priming Kupffer cells to mount an increased inflammatory response including high levels of TNF-α production. In this context, both hepatocyte Rac1/Nox1 and/or Rac1/Nox2 can contribute to ROS production by the hepatocyte, which activates c-Src and NF-κB pathways to induce TNF-α production by the hepatocyte. As in other cell types (28), c-Src in the hepatocyte appears to potentiate H/R-induced NOX-mediated ROS production in the hepatocyte. On the basis of our Kupffer cell-depletion experiments on the various NOX KO backgrounds, we propose that, in vivo, Kupffer cell-derived factors repress a partially NOX2-dependent pathway (labeled “Anti-inflammatory”) that otherwise would inhibit NOX1 activity in the liver. In vivo, this anti-inflammatory pathway may limit NOX1 activity in hepatocytes. In cultured primary hepatocytes (i.e., ex vivo), where this extrinsic factor is absent, NOX1 predominates as the pathway to activate TNF-α following H/R in the absence of other hepatic cell types. In NOX1 KO mice, NOX2 in the hepatocyte compensates for the loss of NOX1, and I/R responses are similar to WT animals. In NOX2 KO mice, NOX1 can still lead to low levels of acute-phase TNF-α production following I/R; however, the amplification of proinflammatory responses by the Kupffer cells is absent since NOX2 is the predominant NOX in this cell type. In Nox1/2 double-KO mice, ROS-dependent signaling that activates much of the TNF-α response following I/R is absent. In WT mice lacking Kupffer cells, a NOX2-dependent anti-inflammatory state is induced by the lack of Kupffer cell-secreted proinflammatory factors, and I/R-mediated TNF-α responses are attenuated. In NOX1 KO animals lacking Kupffer cells, the NOX2-dependent anti-inflammatory state is maintained, whereas Kupffer cell proinflammatory contributions are eliminated, leading to diminished I/R-mediated plasma TNF-α levels. In NOX2 KO Kupffer cell depleted animals, the NOX2-dependent anti-inflammatory state is no longer present and this transitions to a proinflammatory state raising both the baseline and I/R-induced TNF-α production by hepatocytes and potentially other non-Kupffer hepatic cell types. In this case (the absence of NOX2 and Kupffer cells), NOX1 activity may increase in the hepatocyte and/or other cell types, leading to enhanced NOX1-dependent production of TNF-α. In NOX1/2 double-KO animals depleted for Kupffer cells, the proinflammatory state caused by absence of both NOX2 and Kupffer cells is attenuated since NOX1 is not present to induce TNF-α production.