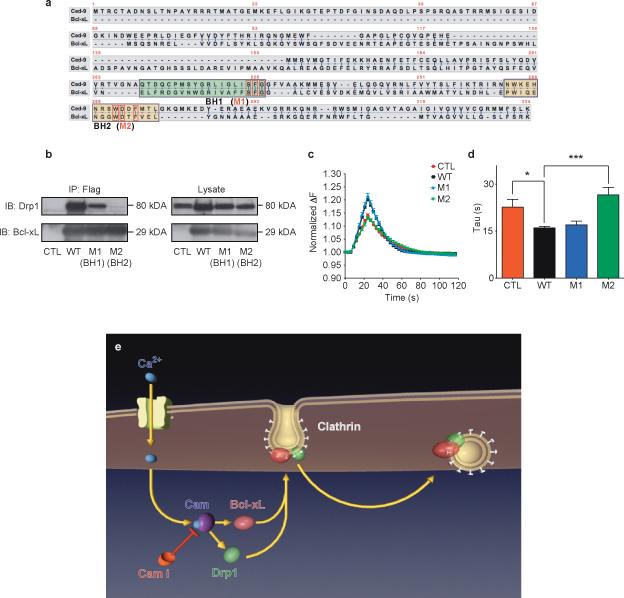
Figure 8. Mutations in BH2 domain of Bcl-xL disrupt physical and functional interaction with Drp1.
a. CED-9 and Bcl-xL protein sequences. Green and yellow boxes specify BH1 and BH2 domains, respectively. Red boxes denote conserved mutated amino acids within each domain.
b. Immunoprecipitation by an anti-FLAG antibody of the wild type and mutant (M1 and M2) FLAG-tagged Bcl-xL proteins. Samples were immunoblotted for Drp1 and Bcl-xL. Lysate studies are shown in the right panel.
c. Normalized (to starting value) fluorescence change of synaptopHluorin puncta in hippocampal neurons expressing indicated constructs before, during and after electrical stimulation (with 100 action potentials, 10 Hz), n=10 puncta each from 3 cells each from one culture.
d. Group data for all experiments shown in c (*p=0.0169; ***p=0.0004).
e. Model showing order of events: 1) Neuronal stimulation leads to calcium influx. 2) Calcium binds to and activates Calmodulin (CaM), which is inhibited by calmidazolium (CaMi). 3) CaM causes the translocation of Bcl-xL and Drp1 to clathrin-coated pits where Bcl-xL binds to and activates Drp1. 4) Drp1 is in part responsible for the formation of normally curved endocytotic vesicles. Knock down or functional inhibition of Bcl-xL, Drp1 or CaM disrupts this process, slowing endocytosis.