Abstract
Major developments in the neural stem cell (NSC) field in recent years provide new insights into the nature of the NSC niche. In this perspective, we integrate recent anatomical data on the organization of the two main neurogenic niches in the adult brain, the ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ), with signaling pathways that control the behavior of NSCs. NSCs in the adult brain stretch into physiologically distinct compartments of their niche. We propose how adult NSCs’ unique morphology may allow these cells to integrate multiple signaling pathways arising from unique locations of their niche.
The fascinating process of developmental tissue growth and morphogenesis is orchestrated by stem cells that contribute to organ maintenance (tissue homeostasis) and repair in the adult. For many years, the brain, with its extraordinary structure, connectivity, complexity, and diversity of cell types, was considered an exception; neural stem cells (NSCs) were thought to be present only during development when this amazing organ is put together. This view began to change with the discovery of adult neurogenesis [for historical perspective, see (Altman, 2011; Nottebohm, 2011)], followed by the identification of cells that in vitro (Ray et al., 1993; Reynolds and Weiss, 1992) and in vivo (Doetsch et al., 1999; Seri et al., 2004; Seri et al., 2001) that can function as NSCs generating neurons, glial cells or both. These discoveries led to a shift in concepts, not only about the potential of the postnatal brain to engage in processes only thought possible in development, but also about the nature of NSCs themselves.
In the adult mammalian brain, NSCs are retained in two regions, the ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ). The V-SVZ, in the walls of the lateral ventricles (Figure 1, upper panel), contains a subpopulation of cells with astroglial properties (B1 cells) that function as NSCs, giving rise to intermediate progenitors (IPCs or transient amplifying progenitors, also known as C cells), which in rodents predominantly generate neurons destined for the olfactory bulb (OB) (For review see; (Kriegstein and Alvarez-Buylla, 2009)). In the SGZ at the interface of the hilus and dentate gyrus (Figure 2, upper panel), NSCs also correspond to astroglial cells, which have a radial process that traverses the granule cell layer. These cells, known by multiple names -- radial astrocytes (Seri et al., 2004; Seri et al., 2001), type-1 progenitors (Filippov et al., 2003) or radial glia-like cells (Bonaguidi et al., 2011) --, generate new dentate granule neurons via IPC1 and IPC2 (also known as type 2a and 2b cells, respectively) (for review see (Zhao et al., 2008)). In both germinal zones, NSCs and IPCs correspond to the primary and secondary progenitors, respectively. Many studies looking at proliferation do not distinguish between primary or secondary progenitors and in these cases we will refer to both collectively as progenitor cells.
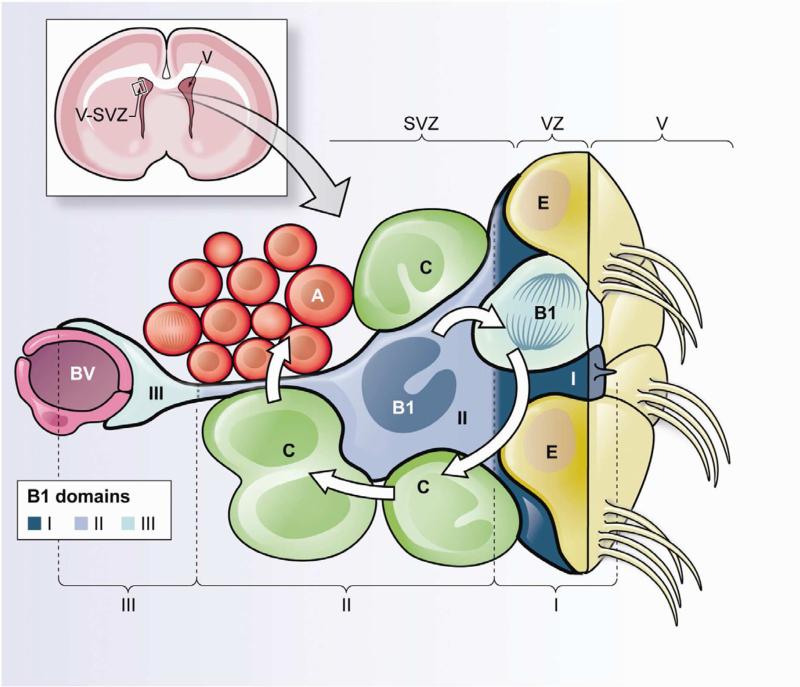
Figure 1. Schematic of the different domains of B1 cells within the adult V-SVZ.
Upper left. Frontal cross-section of the adult mouse brain showing the location of the ventricularsubventricular zone (V-SVZ), where neurogenesis in walls of the lateral ventricles (V) continues throughout life.
Lower panel. Cellular composition of the adult V-SVZ niche and domains of B1 cells. Neural stem cells (NSCs) correspond to type B1 cells (blue). B1 cells are surrounded by multiciliated ependymal cells (E) forming pinwheel-like structures on the ventricular surface. B1 cells give rise to intermediate progenitors (IPCs or C cells, green), which correspond to transit-amplifying cells that divide to generate neuroblasts (Type A cells, red). B1 cells retain epithelial properties, with a thin apical process (containing a primary cilium) that contacts the lateral ventricle (V), and a long basal process ending on blood vessels (BV, purple). Therefore, B1 cells can be subdivided into three domains. Domain I (proximal or apical, dark blue) contains the primary cilium and is in direct contact with the CSF; within this domain B1 cells can access soluble factors within the CSF and signaling molecules from neighboring ependymal cells. Domain II (intermediate, medium blue) is in close proximity to IPCs, neuroblasts, neuronal terminals and other supporting cells; cell-cell interactions between B1 cells and their progeny could occur within this compartment. Domain III (distal, light blue) comprises a basal process ending in a specialized end-foot that contacts blood vessels; blood-borne factors and endothelial-derived factors may act on B1 cells in this domain.
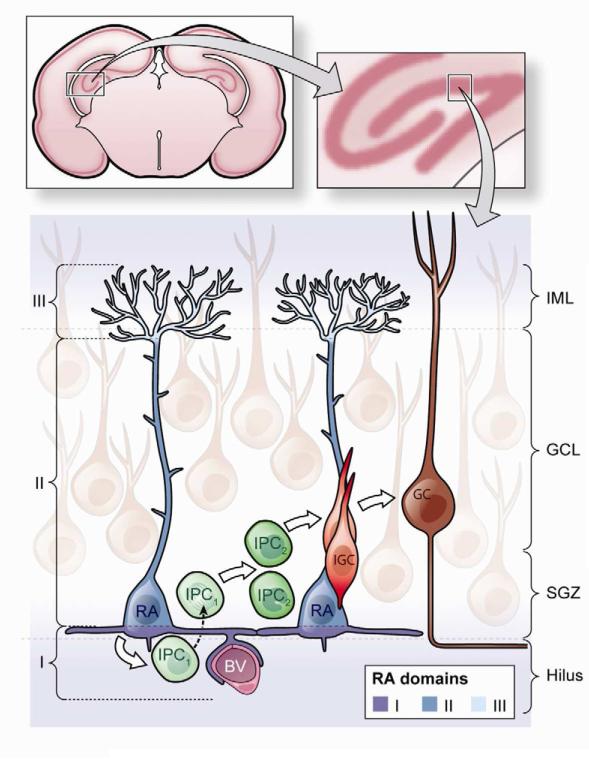
Figure 2. Schematic of the different domains of SGZ radial astrocytes.
Upper panel. Frontal cross-section of the adult mouse brain showing the hippocampal formation (left). The insert shows a higher magnification indicating the location of the dentate gyrus (right).
Lower panel. Cellular composition of the adult dentate gyrus and domains of SGZ radial astrocytes. Radial astrocytes (RA, also known as type 1 cells (blue)) give rise to intermediate progenitors (IPCs, green), which progressively (via IPC1 and IPC2 (type 2a and type 2b cells)) differentiate into immature granule cells (IGCs (type 3 cells), red). Mature granule cells (GCs, brown) send an axon parallel to the SGZ into the hilus, while their dendrites branch into the ML. Radial astrocytes are polarized cells with their cell body in the SGZ, a long main shaft that traverses through the granule cell layer (GCL) and then branches diffusely in the inner molecular layer (IML). Here we subdivide radial astrocytes into three domains. Domain I (proximal, dark blue) faces the hilus, harbors a primary cilium, contacts with blood vessels (BV, purple) and through lateral processes, neighboring radial astrocytes. Factors derived from blood, endothelial cells and neighboring radial astrocytes act on NSCs within this domain. Domain II (intermediate, medium blue) contains the cell body and the main shaft. This part of the cell interacts closely with IPCs and GCs; this domain allow specific cell-cell interactions of NSCs with their progeny and detection of local neural activity and signaling from GCs. Domain III (distal, light blue) contacts other glial cells, axons, and synaptic terminals in the inner molecular layer; NSCs may detect levels of neural activity from Mossy cells and other neurons within this compartment.
The adult V-SVZ NSCs in the walls of the lateral ventricles differ significantly in location and structure from those in the hippocampal SGZ. Unlike B1 cells in the V-SVZ, which like many embryonic neural stem cells lie next to the ventricle and have processes that contact the cerebrospinal fluid (CSF), dentate radial astrocytes are found deeper in the brain parenchyma, away from the walls of the ventricle and surrounded by neurons and other glial cells. Yet, B1 cells and radial astrocytes share some key features. Both express astroglial markers and have ultrastructural characteristics of astrocytes (Kriegstein and Alvarez-Buylla, 2009). Most importantly, they both have long processes that allow them to reach into compartments of the niche far away from where the cell bodies reside (Figures 1 and 2). Through some of these processes NSCs make contact with the vasculature, which in both germinal regions plays key roles in their regulation. Therefore, NSCs are in contact with unique compartments of their niche and this may determine whether they remain quiescent or are induced to proliferate.
A large number of studies during the last decade provide important new insights about the nature of adult NSCs and IPCs and about the microenvironment that surrounds them. In this perspective, we aim to connect the recent literature providing insights into the anatomy of the adult neurogenic niches with the emerging knowledge of factors that control the behavior of NSCs (Table I). Organization of adult V-SVZ and SGZ NSCs into distinct domains may ultimately provide an integrative perspective on how adult NSCs are regulated (Figures 1 and 2).
Table 1. Summary of factors involved in the regulation of progenitor cell behavior and putative domains where they could act.
This table summarizes some of the known factors that affect progenitor behavior, although for many factors it is still unclear whether they act directly on NSCs, IPCs, or both. We here propose possible sites of action within the three proposed putative domains of B1 cells (Figure 1) and radial astrocytes (figure 2), the NSCs of V-SVZ and SGZ, respectively.
| B1 cell compartment | Factor | Effect on progenitor cells | References |
|---|---|---|---|
| I. Proximal (apical) | Igf2 Noggin/LRP2 Noggin/Chordin Shh |
Proliferacion ↑ Proliferation ↑ Oligodendrogenesis ↑ Ventral specification |
Lehtinen et al., 2011 Lim et al., 2000, Peretto et al., 2004, Gajera et al., 2010 Colak et al., 2008, Jablonska et al., 2010 Ihrie et al., 2011 |
| II. Intermediate | Notch GABA DBI Dopamine Serotonin |
Maintenance Proliferation ↓ Proliferation ↑ Proliferation ↑ Proliferation/Neurogenesis ↑ |
Imayoshl et al., 2010, Aguirre et al., 2010, Chapouton, 2010 Liu et al., 2005, Fernando et al., 2011 Alfonso et al., 2012 Hoglinger et al., 2004, O'Keeffe et al., 2009, Kim et al., 2010 Banasr et al., 2004 |
| III. Distal (basal) | SDF1 PEDF BTC |
Recruitment to vasculature Proliferation ↑ Proliferation/Neurogenesis ↑ |
Shen et al., 2008 Ramirez-Castillejo et al., 2006, Andreu-Agullo et al., 2009 Gomez-Gaviro et al., 2011 |
| Radial Astrocyte compartment | Factor | Effect on progenitor cells | References |
|---|---|---|---|
| I. Proximal | VEGF primary Cilium |
Proliferation/Neurogenesis ↑ Proliferation ↑ Maintenance |
Licht et al., 2011, Cao et al., 2004 Amador-Arjona et al., 2011 Breunig et al., 2008, Han et al., 2008 |
| II. Intermediate | Notch Noggin/FXR2 BMPs long-term potentiation seizures |
Maintenance Proliferation ↑ Quiescence Proliferation/Neurogenesis ↑ Proliferation/Neurogenesis ↑ |
Lugert et al., 2010, Ables et al., 2010, Ehm et al., 2010 Bonaguidi et al., 2008. Guo et al, 2011 Mira et al., 2010 Bruel-Jungerman et al., 2006 Parent et al., 1997, Ma et al., 2009 |
| III. Distal | GABA Glutamate/NMDAR |
Proliferation ↓ Differentation ↑ Differentation ↑ |
Wang et al., 2005, Tozuka et al., 2005 Deisseroth et al., 2004 |
Upper. Factors that may act on V-SVZ B1 cells. Domain I: Factors released from ependymal cells and present in the CSF. Domain II: Signaling between B1 cells and their progeny or neuronal terminals from other parts of the brain. Domain III: Blood-borne factors and those released from endothelial cells.
Lower. Factors that may act on SGZ radial astrocytes. Domain I: Vascular-derived factors and signaling acting through the primary cilium. Domain II: Factors that could facilitate interaction between radial astrocytes and surrounding cells. Domain III: Neuronal input within the IML.
Domains of adult V-SVZ B1 cells
V-SVZ B1 cells are immersed in a remarkably diverse microenvironment. The highly specialized architecture within this niche implicates both cell-cell interactions and soluble factors as important regulators of NSC behavior. B1 cells retain the basic apical-basal polarity of their predecessors, radial glia. Similar to radial glia and neuroepithelial cells, most, if not all, B1 cells contact the ventricle through small, specialized apical processes that contain a single primary cilium (Mirzadeh et al., 2008; Shen et al., 2008). They also have long basal processes with specialized endings contacting blood vessels (BV). Therefore, adult B1 cells are also part of a VZ and not only a SVZ, hence the new descriptor: V-SVZ (Ihrie and Alvarez-Buylla, 2011). A VZ is retained in many, if not all, adult vertebrates including birds, reptiles, amphibians, and fish (Alvarez-Buylla et al., 1998; Byrd and Brunjes, 2001; Chapouton et al., 2007; Garcia-Verdugo et al., 2002; Goldman and Nottebohm, 1983; Polenov and Chetverukhin, 1993). To better understand how signals within the V-SVZ may be compartmentalized, we propose that V-SVZ B1 cells can be subdivided into three domains: proximal (apical, I.), intermediate (II.) and distal (basal, III.) (Figure 1).
A periscope in the ventricle - B1 cells’ proximal domain
The proximal domain of B1 cells is in direct contact with the ventricle. When viewed en face, from the ventricular side, the rodent V-SVZ is organized as pinwheels; the small apical endings of B1 cells in the center are surrounded by a rosette of ependymal cells with large apical surfaces (Figure 1). Intercellular junctions are found between B1 cells, at B1-ependymal boundaries, and between ependymal cells, and each type has unique ultrastructural characteristics (Mirzadeh et al., 2008). Expression of Ankyrin3, an adaptor protein known to regulate the attachment of membrane proteins (including N-cadherin) to the cytoskeleton, is specifically found in the apical-lateral borders of ependymal cells but not in B1 cells. Its expression is controlled by the ependymal-specific transcription factor FoxJ1. Inactivation of FoxJ1 in the postnatal brain results in decreased Ankyrin3 and reduced neurogenesis (Paez-Gonzalez et al., 2011).
Ependymal cells also help maintain the molecular composition of the apical compartment by propelling the CSF with their multiple motile cilia (Sawamoto et al., 2006). B1 cells with their small apical surface are in direct contact with the CSF, which contains soluble factors that could modulate NSC behavior (Lehtinen et al., 2011; Zappaterra et al., 2007). IGF2 in the adult CSF has been shown to regulate V-SVZ progenitor proliferation (Lehtinen et al., 2011). BMPs, Wnts, SHH, and retinoic acid are also present in the CSF and may modulate the behavior of B1 cells (Huang et al., 2010; Lehtinen et al., 2011). Ependymal cells secrete the BMP antagonist Noggin, which promotes V-SVZ progenitor proliferation and neuroblast generation in vitro and in vivo (Lim et al., 2000; Peretto et al., 2004). Consistently, ependymal expression of LRP2, a receptor that sequesters BMP4, is required for progenitor proliferation and neurogenesis in vivo (Gajera et al., 2010). Yet, there is other evidence indicating that inhibition of BMPs increases oligodendrogenesis at the expense of neurogenesis (Colak et al., 2008) and might be involved in increased glial differentiation upon demyelination (Jablonska et al., 2010).
The proximal domain of B1 cells also harbors a primary cilium that may directly integrate signaling of CSF factors, although such integration remains to be demonstrated. Disruption of IFT88, a protein required for cilia assembly, in GLAST+ cells results in decreased numbers of adult BrdU-label retaining cells (Beckervordersandforth et al., 2010). In contrast, another recent study suggests that depletion of IFT20 in GFAP+ cells at early postnatal stages had only minor effects on neurogenesis in the adult V-SVZ (Amador-Arjona et al., 2011). The primary cilium is essential for transduction of Shh signaling during neural tube development (Wong and Reiter, 2008). It is therefore possible that this organelle may be required for the transduction of Shh signaling observed in specific subregions of the V-SVZ (Ihrie et al., 2011).
Necking with the neighbors - B1 cells’ intermediate domain
Sustained neurogenesis throughout life requires a tight balance between NSCs proliferation and the number of differentiated progeny produced. It has been hypothesized that feedback mechanism must exist to inform NSCs of the number of new neurons already produced. The intermediate domain of B1 cells is in intimate contact with IPCs and neuroblasts allowing direct feedback mechanisms from progeny to NSCs. Canonical Notch signaling is highly active in V-SVZ NSCs and regulates their maintenance (Imayoshi et al., 2010). Conditional depletion of the downstream effector RBPJκ in adult Nestin+ cells leads to a transient increase in IPCs and newborn OB neurons followed by a drastic reduction in NSC numbers that ultimately results in reduced neurogenesis. Thus, Notch signaling might maintain B1 cells by inhibiting the production of IPCs. IPCs express high levels of Ascl1 (Mash1), which is repressed by Hes1, a downstream effector of Notch signaling. Ascl1 in turn is known to promote the expression of Notch ligands (Kopan and Ilagan, 2009), suggesting a possible feedback mechanism via lateral inhibition between IPCs and NSCs by direct cell-cell-contact. Feedback mechanisms may also occur among NSCs as shown in zebrafish (Chapouton et al., 2010). In this vertebrate model, expression of the Notch ligand, DeltaA, in dividing NSCs, activates Notch in neighboring NSCs maintaining quiescence. Although it is unclear how Notch signaling activity is regulated in the murine V-SVZ, Notch ligands are expressed throughout the V- SVZ and Delta1 and Jagged1 expression has been observed in IPCs and neuroblasts (Aguirre et al., 2010; Irvin et al., 2004). This is in agreement with earlier observations in which ablation of IPCs and neuroblasts by the anti-mitotic drug AraC activates NSCs that leads to V-SVZ regeneration (Doetsch et al., 1999). On the other hand, it has been suggested that increased numbers of IPCs due to enhanced EGFR signaling may suppress Notch signaling in NSCs, although the exact mechanism remains unclear (Aguirre et al., 2010). During development, Notch signaling oscillates during interkinetic nuclear migration of radial glia (Shimojo et al., 2008). It is unknown whether Notch signaling oscillates in the adult V-SVZ during cell cycle progression. However, it has been shown that mitotic B1 cells tend to be closer to the ventricular surface (Mirzadeh et al., 2008). Whether changes in the position of B1 cells’ nuclei are associated with different phases of the cell cycle remains to be determined (Shen et al., 2008; Tavazoie et al., 2008).
Neurotransmitters in the V-SVZ also appear to regulate NSC behavior, and this regulation is likely to occur in the intermediate domain where newly generated neuroblasts closely interact with both of their predecessors, B1 cells and IPCs. Neuroblasts spontaneously release the neurotransmitter GABA and induce depolarization of progenitors by activation of functional GABAA-receptors. This, in turn, inhibits progenitor cell cycle progression and neuronal production via epigenetic mechanisms that involves phosphorylation of H2AX (Fernando et al., 2011; Liu et al., 2005). The diazepam binding inhibitor protein (DBI) is secreted into the extracellular space by B1 cells and IPCs, but not neuroblasts, and competes with GABA for binding to its receptor. This leads to decreased inward Cl− currents resulting in increased proliferation of the progenitor population (Alfonso et al., 2012).
V-SVZ progenitors also receive inputs from non-neurogenic regions of the brain. The neurotransmitter dopamine is released into the V-SVZ from terminals of neuronal projections from the substantia nigra. Activation of dopamine D2-like receptors on IPCs increases their proliferation via an EGF-dependent mechanism (Hoglinger et al., 2004; O'Keeffe et al., 2009). Dopamine may also induce IPC proliferation and OB neurogenesis via D3 receptors (Kim et al., 2010). In addition, serotonin release from raphe nuclei neurons into the V-SVZ has been shown to positively modulate neurogenesis, although it remains to be determined if this effect is direct (Banasr et al., 2004).
Stretching out to the vasculature - B1 cells’ distal domain
An extensive vascular plexus runs parallel to the V-SVZ. A specialized end-foot in the basal process conforming the distal domain of B1 cells (Figure 1) allows these NSCs to interact closely with endothelial cells (ECs). Indeed, ECs, and possibly factors derived from the circulation, support proliferation, and self-renewal of V-SVZ progenitors in vitro (Shen et al., 2004). Clusters of dividing B1 cells and IPCs are associated with BVs at regions where the blood-brain barrier appears to be leaky (Tavazoie et al., 2008). Blood-derived factors may directly access B1 cells and IPCs and regulate their proliferation. The chemokine SDF1 is expressed by endothelial and ependymal cells forming a gradient within the V-SVZ (Kokovay et al., 2010). High levels of SDF1 secreted by endothelial cells induce the recruitment of activated B1 cells and IPCs by chemotaxis into the vascular plexus. This effect is mediated by the induction of α6β1-integrin expression in these cell populations. Blockage of α6-integrins in progenitor cells results in the loss of adhesion to the vasculature and proliferation defects in vivo. Interestingly, the transition of IPCs into neuroblasts is accompanied by a decrease in the expression of β1integrin, enabling the differentiating progeny to migrate away from their niche.
Pigment epithelium-derived factor, PEDF, is also secreted by endothelial and ependymal cells in the V-SVZ. PEDF infusion into the lateral ventricles results in a significant increase in the number of BrdU-label retaining cells and in the number of dividing GFAP+ cells (Ramirez-Castillejo et al., 2006). PEDF interacts synergistically with the Notch pathway to regulate self-renewal in vitro by increasing EGFR expression (Andreu-Agullo et al., 2009). More recently, endothelial-derived Betacellulin (BTC), an EGF-like growth factor, has been shown to stimulate progenitor proliferation in vitro. BTC infusion in vivo induces a significant increase in V-SVZ proliferation and neurogenesis (Gomez-Gaviro et al., 2012). Interestingly, the effects of BTC are different from those of EGF, which like BTC induces a burst in progenitor proliferation, but results in reduced neurogenesis and increased oligodendrogenesis (Doetsch et al., 2002; Gonzalez-Perez et al., 2009; Kuhn et al., 1997). This could be explained in part by the observation that BTC can bind and activate ErbB and EGFR receptors present in neuroblasts and progenitor cells, respectively, while EGF mostly acts on IPCs. Notably, V-SVZ NSCs fail to regenerate IPCs and neuroblasts in BTC-null mice following anti-mitotic treatment with AraC (Gomez-Gaviro et al., 2012).
Taken together, the striking morphology of B1 cells allows them to interact with multiple environments. The proximal domain allows B1 cells with their apical surface, including the primary cilium, to receive signals from the CSF and from neighboring ependymal cells. The intermediate domain is likely a site for feedback signaling with IPCs and neuroblasts. B1 cells further access local (endothelial-derived) but also distant (blood-derived) secreted factors with their distal domain. However, some signaling pathways may function in multiple domains of B1 cells. For example, it is likely that Notch signaling, in addition to its function in the intermediate domain mediating interaction of IPCs and NSCs, also plays an important role in the proximal domain of these primary progenitors (e.g. between B1 cells and ependymal cells). It will be interesting to determine if the response of NSCs is tuned to the domain, to the signaling pathway or to both. Real-time imaging of B1 cells may allow future studies to monitor responses within different domains of NSCs and provide a more precise context under which the different domains contribute to specific NSC behavior.
Domains of adult SGZ radial astrocytes
NSCs in the hippocampal SGZ, unlike those in the V-SVZ, are not in contact with the ventricular system. Nevertheless, radial astrocytes are regularly arrayed in the SGZ and along the dentate gyrus and are highly polarized, similar to apical-basal organization observed in radial glia and B1 cells (Kempermann et al., 2003; Seri et al., 2004; Seri et al., 2001). Radial astrocytes also span at least three putative domains. We define the proximal domain as the side of radial astrocytes that faces the hilus and includes contacts with blood vessels, a primary cilium, and lateral processes that frequently contact other radial astrocytes. The intermediate domain includes the cell body and the main shaft through the granule cell layer, where the cells have thin appendages intercalated among mature granule neurons. The distal domain is highly branched and contacts neuronal processes, synapses, and other glial cells in the inner molecular layer. Radial astrocytes may contact blood vessels in multiple compartments, but interactions with the vasculature have been only studied in their proximal domain (Figure 2).
Snooping into the hilus - radial astrocytes’ proximal domain
The SGZ is intimately associated with a rich bed of endothelial cells. Interestingly, active angiogenesis and vascular remodeling occurs in parallel with neurogenesis (Palmer et al., 2000). This is in sharp contrast to the V-SVZ, where endothelial cell division is rare or undetectable (Shen et al., 2008; Tavazoie et al., 2008). Increased angiogenesis is associated with expression of the vascular endothelial growth factor (VEGF), which is also associated with increased progenitor proliferation and neurogenesis (Cao et al., 2004; Licht et al., 2011). However, whether VEGF acts directly on radial astrocytes remains unknown. Notably, physical exercise, a paradigm that induces proliferation of Sox2+ radial astrocytes (Suh et al., 2007), increases VEGF expression (Cao et al., 2004). The vasculature also secretes IGF1 and BDNF, which promote proliferation and differentiation of progenitor cells, respectively (Chen et al., 2005; Llorens-Martin et al., 2009).
The proximal domain of radial astrocytes also harbors a primary cilium, which has been shown to be essential for Shh signaling. Conditional deletion of Ift20, which controls ciliary assembly, in GFAP+ radial astrocytes results in a significant decrease in SGZ IPC proliferation and a concomitant impairment in spatial learning (Amador-Arjona et al., 2011). Notably, disruption of ciliogenesis during development results in decreased dentate gyrus Shh signaling and almost a complete absence of radial astrocytes during postnatal life (Breunig et al., 2008; Han et al., 2008). Similar defects in the establishment of radial astrocytes occur upon mutation of the Smoothened receptor, which is essential for Shh signaling (Han et al., 2008). These results indicate that Shh signaling through the primary cilium is essential for the transition from embryonic to adult NSCs in the SGZ.
Shoulder to shoulder with the progeny - radial astrocytes’ intermediate domain
The intermediate domain of SGZ radial astrocytes, which contains most of the cell body and the main shaft of the radial astrocyte process, contacts IPCs, populations of mature granule neurons, and possibly other neuronal cell types. The majority of IPCs are found next to radial astrocytes’ cell bodies (Kempermann et al., 2003; Seri et al., 2004), in what we here define as part of the intermediate domain. However, there is some evidence that suggest that early IPCs (IPC1) are generated by asymmetric division of the radial astrocytes with a horizontal mitotic plane parallel to hilus (Encinas et al., 2011; Seri et al., 2004). Therefore, these initial IPCs may transiently interact with the proximal domain. However, proliferating IPC1 cells are observed in the intermediate domain suggesting that after they are produced, they rapidly translocate to the intermediate domain next to the radial astrocyte's cell body. Recent work using lineage-tracing experiments of Hes5-expressing NSCs, confirms that Ascl1+ IPC1 proliferate, but they apparently only divide once before converting into IPC2 cells that no longer express Ascl1, but have turned on the early neuronal marker DCX (Lugert et al., 2012). Interestingly, this study suggests that further amplification of the lineage occurs by division of the DCX+/Tbr2+ IPC2 cells.
The high expression of Hes5 (and also RBPJκ) in radial astrocytes indicates active canonical Notch signaling in these NSCs (Ehm et al., 2010; Lugert et al., 2010). IPCs in turn are thought to express the Notch ligand Jagged-1 (Breunig et al., 2007; Lavado et al., 2010). Maintenance of quiescence among radial astrocytes might be controlled through a feedback mechanism by Jagged-1/Notch. Consistently, conditional deletion of RBPJκ in adult GLAST+ SGZ radial astrocytes results in short-term expansion of the IPC pool and reduction in the number of radial astrocytes (Ehm et al., 2010). In contrast, conditional ablation of Notch1 in Nestin+ cells results in a strong reduction in the number of radial astrocytes, but without a transient increase in IPCs (Ables et al., 2010). This difference might be explained by compensatory effects of other Notch receptors or by non-canonical Notch signaling. Notably, the expression of Sox2, a transcription factor essential for maintenance of radial astrocytes (Favaro et al., 2009), is regulated by Notch signaling via RBPJκ, which directly targets the Sox2 promoter (Ehm et al., 2010). Sox2, in turn, inhibits Wnt-mediated activation of the neuronal fate determinant NeuroD, (Kuwabara et al., 2009) and it may directly target Shh expression (Favaro et al., 2009). Thus, reduction in Notch signaling and Sox2 expression may be required for the induction of proneural genes like Ascl1 and NeuroD to stimulate the transition from radial astrocytes to IPCs.
Noggin is expressed in dentate gyrus granule cells, the hilus and SGZ radial astrocytes, and its expression is regulated in a cell-autonomous manner by the RNA-binding protein FXR2 (Bonaguidi et al., 2008; Guo et al., 2011). Physical exercise reduces BMP4 and increases Noggin expression (Gobeske et al., 2009), and overexpression of Noggin increases the number of dividing GFAP+ radial astrocytes (Bonaguidi et al., 2008). This suggests that BMPs inhibit NSC proliferation. Another recent study indicates that BMP controls NSC quiescence in the SGZ (Mira et al., 2010). Non-dividing radial Sox2+ cells show high levels of activated Smad1, a specific mediator of BMP signaling, while dividing progenitors do not. Consistent with a role for BMPs in NSC quiescence, Noggin infusion results in an initial increase in neuronal production, and a concomitant reduction of Sox2+ progenitors and neuronal production at later time points. Conditional ablation of the receptor BMPRIa or of Smad4 also results in decreased quiescence among NSCs.
The shaft of radial astrocytes in the intermediate domain is also intimately associated with mature granule neurons. Direct contact or exchange of signals with neurons may contribute to radial astrocyte regulation. It is possible that radial astrocytes may be able to sense neighboring network activity associated with the column of granule neurons surrounding their radial shaft, possibly through neurotransmitter-mediated spillover or extracellular potassium. Neuronal activity, induced for example by learning and memory, or seizures (Inokuchi, 2011), may directly activate proliferation of NSCs in the dentate gyrus. Hippocampal induction of long-term potentiation (LTP), a widely studied mechanism associated with memory formation (Lynch, 2004), results in increased SGZ proliferation and neuronal differentiation (Bruel-Jungerman et al., 2006). General activation of granule neurons following seizures results in increased SGZ neurogenesis, although the precise mechanisms of this effect remain elusive (reviewed in (Kokaia, 2011)). One study suggests that Shh signaling rapidly increases following electroconvulsive treatment (ECT) and that Shh may be required for seizure induced SGZ proliferation (Banerjee et al., 2005). Interestingly, after ECT, granule neurons transiently express high levels of the DNA repair protein Gadd45b, a process that requires NMDA-type glutamate receptors (NMDARs) (Ma et al., 2009). Loss of Gadd45b expression partially blocks ECT-induced SGZ progenitor proliferation. This study also shows that Gadd45b induces the expression of genes known to modulate neurogenesis such as BDNF and FGF1 by DNA-demethylation at promoter regions. Seizures result in non-physiological high levels of neuronal activity. Whether physiological levels of neuronal firing, possibly in a localized manner, affect individual radial astrocyte proliferation remains to be determined. Neuronal activity may also exert effects on neurogenesis through the distal or proximal domains of radial astrocytes.
Hanging on wires - radial astrocytes’ distal domain
In addition to a shaft that contacts granule neurons, radial astrocytes have an elaborate and extensive set of thin branches and lamellae in the inner molecular layer. Little is known about the exchange of signals that take place here, but it would be surprising for such an elaborate terminal arbor of the radial process not to function in the regulation of these NSCs. The inner molecular layer of the dentate gyrus receives GABAergic and glutamatergic inputs from hilar interneurons and Mossy cells, respectively (Forster et al., 2006).
Several studies have investigated the role of neurotransmitters in the regulation of SGZ neurogenesis. There is evidence that GABA promotes SGZ differentiation but it remains unknown whether GABAergic inputs have a direct effect on the proliferation of SGZ progenitor cells, as shown for the V-SVZ. GABA does not seem to stimulate radial astrocyte responses directly, but it induces depolarization of IPCs resulting in increased Ca2+ influx and enhanced NeuroD expression. This, in turn, inhibits progenitor proliferation and promotes neuronal differentiation (Tozuka et al., 2005; Wang et al., 2005). Electrical stimulation of the perforant pathway activates granule cells and GABAergic interneurons, and directly induces inward GABAergic currents in IPCs. GABAergic terminals, containing vesicular GABA transporter (VGAT), are closely associated with IPCs (Tozuka et al., 2005). Recent studies have shown that co-release with SDF1 facilitates GABA transmission from local GABAergic basket interneurons (Bhattacharyya et al., 2008; Kolodziej et al., 2008). These findings suggest that SGZ progenitors receive functional inputs from hippocampal GABAergic interneurons that promote neuronal differentiation, but it remains unknown if this is localized uniquely to the distal domain.
Glutamate also induces depolarization of IPCs, inhibits expression of Hes1 and Id2, and increases expression of NeuroD. This process is regulated by voltage-gated Ca2+ channels and is mediated through NMDARs (Deisseroth et al., 2004). However, functional NMDARs are not detectable in SGZ progenitor cells in tissue slices (Tozuka et al., 2005). It will be important to determine if the response of radial astrocytes to glutamate vary depending on the regions of these cells exposed to this neurotransmitter.
In summary, the three domains of radial astrocytes intermix with three anatomical layers: the SGZ, the granule cell layer, and the inner molecular layer. The proximal domain in the SGZ has a primary cilium and interacts with the vasculature. Similar to V-SVZ B1 cells the intermediate domain of radial astrocyte directly interacts with their progeny. The intermediate and distal domains spanning a long appendage and branches in the inner molecular layer may expose radial astrocytes to neuronal networks and their level of activity. The domain organization of NSCs may help explain how radial astrocytes within the dentate gyrus might be able to integrate local activity from granule neurons right next to the primary shaft of these cells versus more widespread inputs arising from parallel fibers in the inner molecular layer and the hilus. There likely exists a local topographic organization to regulate neurogenesis at the level of individual radial astrocytes. This remains an interesting question for future research.
Self-renewal of adult neural stem cells and age related changes
The above suggests that domains in NSCs are tuned to specific compartments within their niche. In order to determine how NSCs integrate this information, the behavior of these cells in vivo needs to be understood. However, the patterns of proliferation of adult NSCs remain unknown or are highly controversial. Self-renewal is considered a defining property of stem cells and important for their long-term retention in adults. Thus, a major question is whether adult NSCs have this classical property of somatic stem cells. NSCs were initially identified in vitro by their ability to generate free-floating aggregates (neurospheres) in response to growth factors (Weiss et al., 1996). So far, most evidence for self-renewal is based on these in vitro assays. A major limiting factor to study NSC behavior in vivo is the lack of markers that exclusively identify these primary progenitors (Kriegstein and Alvarez-Buylla 2009). Thus, although BrdU-label retaining experiments in vivo suggest the presence of cells with stem cell characteristics (Bonaguidi et al., 2008; Chiasson et al., 1999; Doetsch et al., 1999), self-renewal has not been directly demonstrated in vivo in the V-SVZ and this issue remains controversial in the SGZ (Bonaguidi et al., 2011; Encinas et al., 2011; Lugert et al., 2012).
Encinas and collaborators (Encinas et al., 2011) suggest that SGZ NSCs do not self-renew but are consumed with age (the “disposable stem cell” hypothesis). In this scenario, activated radial astrocytes undergo up to three sequential asymmetric divisions that generate IPCs. The NSCs then terminally differentiate into mature astrocytes. This study also suggests that IPCs divide multiple times, which is in contrast to earlier estimations of one to two rounds (Seri et al., 2004). Contrary to the disposable stem cell hypothesis and the depletion of NSCs with time, two other recent studies indicate that stable populations of radial astrocytes are maintained in the SGZ for extended periods of time. Lugert et al. (Lugert et al., 2012) finds that a cohort of Hes5 expressing NSCs, which contributes continually to neurogenesis over this time, remains in the SGZ for up to 100 days, suggesting some level of self-renewal. Contrary to the finding in Encinas et al, lineage tracing of these Hes5+ NSCs do not reveal an increase in the generation of mature astrocytes during this period and the stoichiometry of labeled NSCs, IPCs, and neurons produced also suggests limited IPC amplification. Bonaguidi et al. (Bonaguidi et al., 2011) have followed the lineage of individual Nestin+ radial astrocytes in the SGZ and observed that a small number of these NSCs self-renew symmetrically with a larger population self-renewing asymmetrically. SGZ NSCs are multipotent generating both astrocytes and neurons, while others are unipotent and generated only neurons or astrocytes, therefore suggesting that SGZ NSCs are heterogeneous in their behavior (Bonaguidi et al., 2011). This study also suggests that SGZ IPCs divide up to five times before differentiating into young neurons. Interestingly, asymmetrically dividing radial astrocytes generate a highly proliferative IPC and a radial astrocyte, which, in contrast to the disposable model, returns to a quiescent state (Bonaguidi et al., 2011). Thus, the quiescent state of radial astrocytes may be reversible, with multiple cycles of activation and quiescence. Interestingly, upon conditional PTEN deletion, radial astrocytes differentiate into postmitotic astrocytes after a transient increase in symmetric self-renewing divisions. Irrespective of whether adult NSCs self renew or are consumed, it is clear that neurogenesis in adult V-SVZ and SGZ decrease with age (Conover and Shook, 2011; Jessberger and Gage, 2008) and it will be interesting to see how this controversy resolves to understand how the behavior of NSCs is regulated and changes with aging.
Aging is associated with a decline in cognitive function, impairments in learning and memory, and higher susceptibility to neurodegenerative disorders, a process that is paralleled by the reduction in SGZ neurogenesis (Jessberger and Gage, 2008). Interestingly, this decline may in part be due to changes in factors present in the circulation (Villeda et al., 2011). In heterochronic parabionts, where the vasculature of young and aged mice is surgically joined, the aged milieu decreases neurogenesis in young adults whereas the young milieu has some rejuvenating effects on the aged SGZ. The chemokine CCL-11 is increased in the plasma and CSF of elderly humans and has been suggested as one of the molecules that could decrease SGZ proliferation. Wnt3, which is secreted from hippocampal astrocytes and promotes neuronal differentiation by activation of NeuroD and DCX expression, decreases with age (Kuwabara et al., 2009; Okamoto et al., 2011). Recent data further suggest that the age-related decline in SGZ neurogenesis is due to transition of radial astrocytes into quiescence rather than a loss of the stem cell population. In line with this, and contrary to the depletion of NSCs during aging, physical exercise and seizures, both known to promote neurogenesis in the young adult hippocampus, can (partially) reverse the age-induced changes (Kronenberg et al., 2006; Lugert et al., 2010; Okamoto et al., 2011). Thus, the quiescent state might remain reversible in the aged SGZ. However, whether activation of quiescent stem cells in the aged SGZ is only transient and whether it might lead to subsequent stem cell exhaustion is unknown. On the other hand, activation of quiescent cells itself might not be sufficient to restore neurogenesis (Kronenberg et al., 2006; Rao et al., 2008).
Neurogenesis also declines in an age-dependent manner in the V-SVZ. It has been suggested that aging leads to impairments of fine odor discrimination in mice due to a decrease in the number of newly generated interneurons between 2 and 24 months of age (Enwere et al., 2004). This is accompanied by a diminution in EGFR signaling, proliferation and thinning of the V-SVZ. Some astrocytes acquire ependymal features like 9+2 motile cilia and apical placement of mitochondria and are incorporated into the ependymal layer (Conover and Shook, 2011; Luo et al., 2006). Interestingly, only the dorsolateral aspect of the V-SVZ remains neurogenic in aged mice. This might affect the generation of specific neuronal subtypes in the aged brain, since V-SVZ NSCs are heterogeneous, generating at least six different subtypes of OB interneurons depending on their position along dorsal-ventral and anterior-posterior axes (Alvarez-Buylla et al., 2008; Merkle et al., 2007).
While the number of label-retaining and proliferating cells in vivo seems to decline during aging, the percentage of proliferating cells does not (Bouab et al., 2011). Similarly, the number of V-SVZ neurosphere-forming cells declines with age, but Nestin+ cells appear to be equally competent of producing neurospheres (Ahlenius et al., 2009; Enwere et al., 2004). Cell intrinsic factors like mTERT, p16, and p21 have been shown to contribute to the decrease in neurogenesis associated with aging (Conover and Shook, 2011). Deficiency of p21 increases the numbers of BrdU-label retaining cells in young adult V-SVZ but leads to exhaustion of these cells in old mice (Kippin et al., 2005). It is not clear whether the age related decline in V-SVZ neurogenesis is due to terminal differentiation, a limited intrinsic capacity of NSCs to self-renew and/or molecular and cellular changes in the microenvironment suppressing proliferation and/or promoting senescence or quiescence.
Cell-autonomous quiescence might be a mechanism to prevent tumor formation by minimizing the risk of accumulated mutations and DNA damage. This requires a tight balance of expression of proto-oncogenes and tumor suppressors. For example, the proto-oncogene Bmi1 promotes self-renewal by repressing the tumor suppressors p21, p16, and p19 (Fasano et al., 2009; Favaro et al., 2009; Molofsky et al., 2006). In the aged SVZ, increased levels of p16 suppress stem cell activity but possibly protect from tumor formation. Indeed, it has been shown that adult V-SVZ NSCs could serve as cells of origin for some gliomas (Jacques et al., 2010). Interestingly, p16 is frequently lost in glioblastoma (Furnari et al., 2007; Wiedemeyer et al., 2008).
In sum, it remains unclear whether NSCs self-renew in vivo or are consumed with age, and whether extrinsic factors - within the niche or systemic - decrease NSC proliferation in the aged brain. These are important questions to understand how different compartments in the niche affect the behavior of NSCs. Unlike other tissues where robust regenerative responses can be mounted even in advanced age, neurogenesis in the V-SVZ and SGZ decreases with age. Little is known about how the different compartments of the niche are altered with aging. The integrity of B1 cell proximal domain is likely affected during aging in the ventral V-SVZ. Ventral stenosis, with loss of ependymal cells and fusion of the lateral and medial walls in old mice, has been associated to decrease neurogenesis in this ventral region (Luo et al., 2006). How NSC morphology and signaling within NSC domains is affected with age remains an interesting area for future research.
Closing remarks
Adult NSCs (B1 astrocytes in V-SVZ and radial astrocytes in SGZ) have processes that most likely allow them to sample specific compartments of their niche. Therefore, these NSCs can respond to both local factors next to their cell body as well as signals derived farther away such as those around blood vessels, the ventricles, or neuronal plexus. Moreover, NSC domains allow them to receive signals that are not accessible to other cells within the niche (e.g. CSF-borne factors may not be available to IPCs or neuroblasts within the V-SVZ niche). While it has been shown that NSCs respond to multiple factors, complete insights into NSC behavior will require a better understanding of the compartmentalized signaling that occurs in vivo. How B1 cells and radial astrocytes are induced to proliferate or to remain quiescent likely depends on the subcellular integration of multiple signaling pathways arising from the primary cilium, the end feet around blood vessels, the cell body, and their main or lateral processes. Thus, NSCs bridge multiple locations of the niche allowing them to receive a broad spectrum of signals which combined may orchestrate their behavior. While we have reviewed emerging evidence suggesting how some major signaling pathways may act primarily through one of the proposed domains, as mentioned above, it is entirely possible that the same signaling pathway could also act on other parts of the same cell. The response could differ depending on the region receiving the signal highlighting the relevance of studying NSCs within their niche. Real time imaging of intracellular signaling and simultaneous observation of the resulting NSCs behavior may provide important new insights on the basic regulation of the primary progenitors, which make adult neurogenesis possible.
Acknowledgments
The authors thank Robert A. Lindquist, Timothy R. Stowe, Shawn F. Sorrells and Cheuk Ka Tong for critical discussion of the review. Special thanks also to Kenneth X. Probst for preparation of the illustrations. Due to limited space of this review, we could not cover all the exciting contribution to the field. The Alvarez-Buylla laboratory is funded by the US National Institute of Health (NS28478 and HD32116) and the John G. Bowes Research Fund. A.A.-B. is the Heather and Melanie Muss Endowed Chair of Neurological Surgery at UCSF. L.C.F. is supported by the Helen Hay Whitney Foundation, K.O. by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell. 2012;10:76–87. doi: 10.1016/j.stem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Altman J. The Discovery of Adult Mammalian Neurogenesis. In: Seki T, Sawamoto K, Parent JM, Alvarez-Buylla A, editors. Neurogenesis in the Adult Brain I: Neurobiology. Springer; Tokyo: 2011. pp. 3–46. [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. 2011;31:9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Banerjee SB, Rajendran R, Dias BG, Ladiwala U, Tole S, Vaidya VA. Recruitment of the Sonic hedgehog signalling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur J Neurosci. 2005;22:1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, Stempfhuber B, Kirchhoff F, Hirrlinger J, Haslinger A, Lie DC, et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouab M, Paliouras GN, Aumont A, Forest-Berard K, Fernandes KJ. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience. 2011;173:135–149. doi: 10.1016/j.neuroscience.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. In Proc Natl Acad Sci USA. 2008:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Skupien P, Hesl B, Coolen M, Moore JC, Madelaine R, Kremmer E, Faus-Kessler T, Blader P, Lawson ND, et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30:7961–7974. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, Van der Kooy D. Adult Mammalian Forebrain Ependymal and Subependymal Cells Demonstrate Proliferative Potential, but only Subependymal Cells Have Neural Stem Cell Characteristics. JNeurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Götz M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Shook BA. Aging of the Subventricular Zone Neural Stem Cell Niche. Aging Dis. 2011;2:149–163. [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, et al. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano CA, Phoenix TN, Kokovay E, Lowry N, Elkabetz Y, Dimos JT, Lemischka IR, Studer L, Temple S. Bmi-1 cooperates with Foxg1 to maintain neural stem cell self-renewal in the forebrain. Genes Dev. 2009;23:561–574. doi: 10.1101/gad.1743709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andang M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci U S A. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Forster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7:259–267. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Gotz M, Kempermann G, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4:e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. ProcNatlAcadSciUSA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gaviro MV, Scott CE, Sesay AK, Matheu A, Booth S, Galichet C, Lovell-Badge R. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc Natl Acad Sci U S A. 2012;109:1317–1322. doi: 10.1073/pnas.1016199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zhang L, Christopher DM, Teng ZQ, Fausett SR, Liu C, George OL, Klingensmith J, Jin P, Zhao X. RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron. 2011;70:924–938. doi: 10.1016/j.neuron.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, Litingtung Y, Chiang C. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A. 2010;107:8422–8427. doi: 10.1073/pnas.0911838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, Kriegstein AR, Alvarez-Buylla A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi K. Adult neurogenesis and modulation of neural circuit function. Curr Opin Neurobiol. 2011;21:360–364. doi: 10.1016/j.conb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Irvin DK, Nakano I, Paucar A, Kornblum HI. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J Neurosci Res. 2004;75:330–343. doi: 10.1002/jnr.10843. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, C OM, Naumann H, Alvarez-Buylla A, Brandner S. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2010;29:222–235. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Gage FH. Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging. 2008;23:684–691. doi: 10.1037/a0014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kim Y, Wang WZ, Comte I, Pastrana E, Tran PB, Brown J, Miller RJ, Doetsch F, Molnar Z, Szele FG. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem. 2010;114:750–760. doi: 10.1111/j.1471-4159.2010.06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia M. Seizure-induced neurogenesis in the adult brain. Eur J Neurosci. 2011;33:1133–1138. doi: 10.1111/j.1460-9568.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, Odemis V, Hollt V, Stumm R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. JNeurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, D. TA, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. Noggin Antagonizes BMP Signaling to Create a Niche for Adult Neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15:134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lugert S, Vogt M, Tchorz JS, Muller M, Giachino C, Taylor V. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat Commun. 2012;3:670. doi: 10.1038/ncomms1670. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural Stem Cells Confer Unique Pinwheel Architecture to the Ventricular Surface in Neurogenic Regions of the Adult Brain. In Cell Stem Cell. 2008:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F. Song Learning in Birds Offers a Model for Neuronal Replacement in Adult Brain. In: Seki T, Sawamoto K, Parent JM, Alvarez-Buylla A, editors. Neurogenesis in the Adult Brain I: Neurobiology. Springer; Tokyo: 2011. pp. 47–84. [Google Scholar]
- O'Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, Garcia-Verdugo JM, Kuo CT. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71:61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular Niche for Adult Hippocampal Neurogenesis. Journal of Comparative Neurology. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Peretto P, Dati C, De Marchis S, Kim HH, Ukhanova M, Fasolo A, Margolis FL. Expression of the secreted factors noggin and bone morphogenetic proteins in the subependymal layer and olfactory bulb of the adult mouse brain. Neuroscience. 2004;128:685–696. doi: 10.1016/j.neuroscience.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Polenov AL, Chetverukhin VK. Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. II. Types of neuronal cells produced. Cell Tissue Res. 1993;271:351–362. doi: 10.1007/BF00318622. [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus. 2008;18:931–944. doi: 10.1002/hipo.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. ProcNatlAcadSciUSA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell. 2008:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2(+) Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell. 2007:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem Cell. 2008:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, Van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Wiedemeyer R, Brennan C, Heffernan TP, Xiao Y, Mahoney J, Protopopov A, Zheng H, Bignell G, Furnari F, Cavenee WK, et al. Feedback circuit among INK4 tumor suppressors constrains human glioblastoma development. Cancer Cell. 2008;13:355–364. doi: 10.1016/j.ccr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappaterra MD, Lisgo SN, Lindsay S, Gygi SP, Walsh CA, Ballif BA. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res. 2007;6:3537–3548. doi: 10.1021/pr070247w. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]