Figure 1.
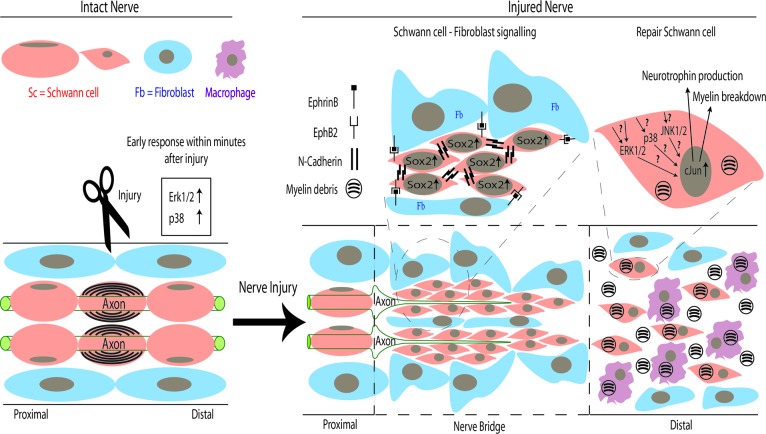
Schematic of repair processes following peripheral nerve transection. In intact mature nerve, differentiated Schwann cells myelinate the large-diameter axons. Following transection injury, ERK1/2 and p38 mitogen-activated protein kinase signaling rapidly increases within Schwann cells distal to the site of injury and causes them to proliferate and take on an undifferentiated “repair” or Büngner cell phenotype. In the distal stump of the nerve, this transdifferentiation of Schwann cells is dependent upon the transcription factor cJun, which directs both the breakdown of myelin and the production of neurotrophins, such as glial-derived neurotrophic factor, brain-derived neurotrophic factor, and Artemin [1]. Following nerve transection, Schwann cells and nerve fibroblasts proliferate and migrate from proximal and distal nerve stumps to form a nerve bridge. Within this bridge, EphrinB-EphB2 signaling between fibroblasts and Schwann cells results in elevation of Sox2, which causes relocalization of N-cadherin to the surface of the Schwann cells. The resulting sorting of Schwann cells into cords in the bridge allows regenerating axons to cross the nerve bridge and reach the distal stump of the nerve [20]. Abbreviations: Erk1/2, extracellular-regulated kinase 1/2; Fb, fibroblast; Sc, Schwann cell.