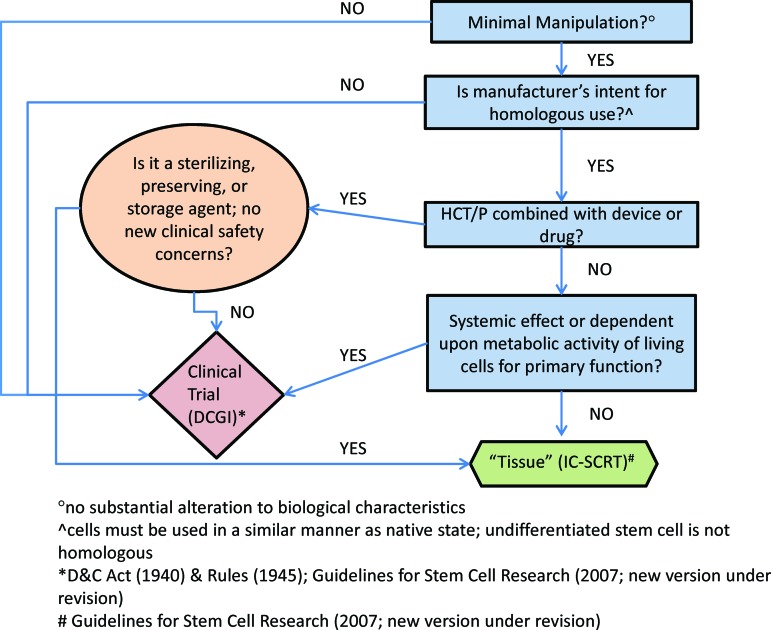
Figure 3.
Suggested classification criteria for a Somatic Cell Therapy Product in India, based on the simplified stratification strategy used by the Food and Drug Administration. If a cell-based therapy meets four criteria, it is regulated as a tissue (green), similar to 361 HCT/P regulations in the United States. If a cell-based therapy fails to meet any of these four criteria, it is regulated as a drug (pink), similar to 351 HCT/P regulations in the United States. Abbreviations: D&C, Drugs and Cosmetics Act; DCGI, Drug Controller General of India; HCT/P, human cells and tissue products; IC-SCRT, Institutional Committee for Stem Cell Research and Therapy.