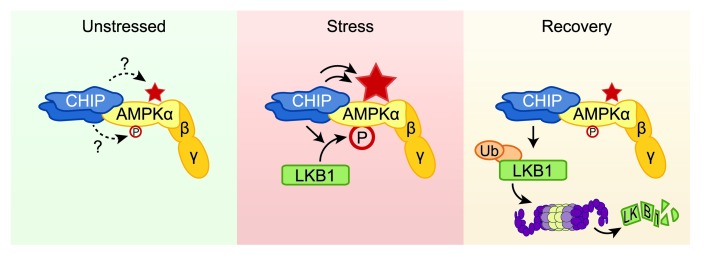
Figure 6. Proposed physiological model for the CHIP-dependent effects on the regulation of AMPK function.
In unstressed conditions, CHIP forms a complex that contains the AMPK holoenzyme; however, the consequence of this interaction is not understood, as CHIP does not appear to directly affect either baseline AMPK activation status, depicted by phosphorylation at α-T172 (P), or baseline AMPK activity (indicated by the star). In cell stress conditions, such as oxidative stress and cardiac pressure overload, or in the presence of AMPK agonists, CHIP is necessary for LKB1-mediated activation of AMPK, as observed by increased phosphorylation of the α (catalytic) subunit, which leads to an increase in AMPK activity. In addition to increasing LKB1-mediated phosphorylation of AMPK, in cell-free systems, CHIP robustly increases AMPK activity independent of phosphorylation, acting as a chaperone of AMPK, amplifying the positive effect of CHIP on AMPK activity. CHIP may also play a key role in the recovery phase in returning the cell to metabolic homeostasis. CHIP mediates polyubiquitination of LKB1, leading to degradation of LKB1 via a proteasome-dependent mechanism that may contribute to restoring AMPK activation status to baseline levels.