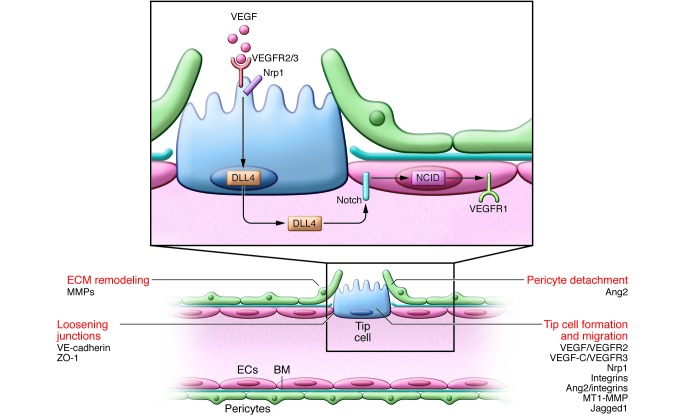
Figure 1. Initial steps of tip cell selection.
Vascular sprouting is initiated by proangiogenic factors (e.g., VEGF). ECs at the leading edge of the vascular sprout extend filopodia and migrate toward angiogenic signals. VEGF activates VEGFR2 to stimulate tip cell migration. The coreceptor Nrp1 complexes with and enhances VEGFR2 signaling. ECs become either the migratory vessel-leading tip cell or the proliferating stalk cell, but their phenotype is fluid; Notch regulates this specification. ECs with activated VEGFR2 signaling compete for the tip cell position by increasing their expression of DLL4, which binds to Notch receptors on neighboring ECs, releasing the transcription regulator NICD. NICD transcriptionally downregulates VEGFR2 and Nrp1 expression while increasing VEGFR1, a VEGF trap, thus enhancing the stalk cells’ unresponsiveness to VEGF. The tip cell is not a fixed position, and fluidity at the front occurs depending on the VEGFR1/VEGFR2 ratio. Tip cell migration requires BM degradation (in part due to MMP), EC junction loosening (caused by VE-cadherin, ZO-1, and others), and pericyte detachment (regulated by Ang2). VEGF increases the permeability of the vessel, allowing the extravasation of plasma proteins (e.g., fibronectin and fibrinogen) that are deposited as a provisional matrix layer while the preexisting interstitial matrix is remodeled by proteases; these events enable tip cell migration. Key molecular players discussed in this review and elsewhere (5, 132) are indicated.