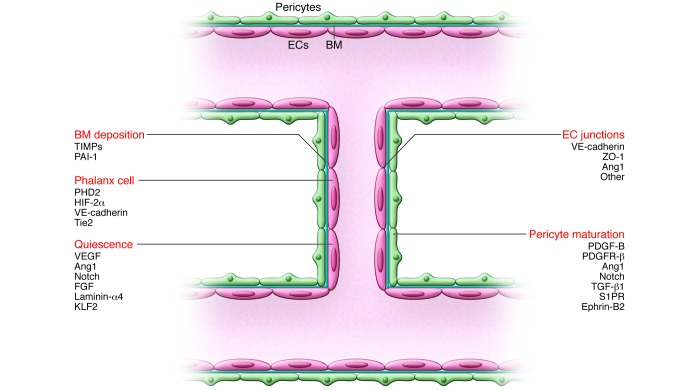
Figure 3. Maturation through resolution of quiescent phalanx cells.
Once fusion has occurred, a connected lumen is formed to allow blood flow through the new vessel. This perfuses the hypoxic tissue, and the resultant oxygen and nutrient delivery leads to decreased levels of angiogenic signals, inactivation of EC oxygen sensors, and increased proquiescent molecules that lead to EC quiescence. Establishment of the blood flow remodels vessel connections, which are regulated by the shear stress–responsive transcription factor KLF2. ECs resume a quiescent phalanx phenotype in a tightly apposed monolayer with a streamlined surface that conducts the blood flow and regulates tissue perfusion. Perfusion induces vascular maturation by reestablishment of cell-cell junctions, pericyte maturation, and BM deposition. Autocrine and paracrine signaling from ECs and surrounding support cells by VEGF, FGF, Ang1, and Notch, among others, maintain a quiescent EC phenotype and protect the vessel from environmental stresses. Reduced growth factor signaling can lead to vessel retraction and EC apoptosis. Once stabilized and matured, the vessel forms a barrier between the blood and surrounding tissue, controlling the exchange of fluids and solutes. Key molecular players discussed in this Review and elsewhere (5, 132) are indicated.