Summary
We assessed trends in the relative prevalences of HIV-1, HIV-2 and dual HIV-1/HIV-2 infection in 10,321 women attending outpatient clinics in Senegal between 1990 and 2009. The relative prevalence of HIV-1 (defined as the proportion of seropositive subjects having HIV-1) rose sharply from 38% in 1990 until 1993 (P < 0.001), whereupon it continued to rise, but at a slower rate, reaching 72% of HIV infections in 2009. As compared with HIV-1, the relative prevalence of HIV-2 decreased sharply from 54% in 1990 until 1993 (P < 0.001) and continued to decrease at a slower rate through 2009. The relative prevalence of dual infection, as compared with HIV-1, was stable from 1990 to 1993, but decreased slightly thereafter (P < 0.001). These study findings indicate that during the early 1990s, the relative prevalence of HIV-1 increased markedly, while the relative prevalence of HIV-2 decreased and the relative prevalence of dual infection remained stable in Senegal. From 1993 to 2009, the relative prevalence of HIV-1 increased at a slower rate, while the relative prevalences of HIV-2 and dual infection decreased. These results confirm trends in HIV prevalence observed in other West African populations and provide a critical update on HIV transmission risk among women in Senegal.
Keywords: HIV, epidemiology, HIV-1, HIV-2, trends, seroprevalence, Africa, Senegal, dual infection
INTRODUCTION
Worldwide, the majority of the ~34 million people living with HIV are infected with HIV type 1.1 In West Africa, however, HIV type 2 is endemic and HIV-1 was more recently introduced;2–5 this has resulted in the presence of HIV-1/HIV-2 dual infection in addition to single infection with one of the two types. Relative to HIV-1, HIV-2 is associated with a slower rate of progression to AIDS6,7 and less efficient vertical and sexual transmissions, which may be related to the lower plasma8–12 and genital tract11,13 viral loads that characterize HIV-2. Although less research has been conducted in dually infected individuals, some data suggest that dual infection progresses at a rate comparable with that of HIV-1 mono-infection.14–17
Over the past 20 years, an increase in the prevalence of HIV-1 and a decrease in the prevalence of HIV-2 has been reported in several West African countries, including Guinea-Bissau,18–21 the Ivory Coast22 and the Gambia.23,24 In Senegal, research on the trends in HIV prevalence has been limited and results have been mixed. A 20-year study of commercial sex workers (CSWs) in Dakar provided evidence of a divergence in the prevalence of HIV-1 and HIV-2,25 but sentinel surveillance performed from 1989 to 1996 did not show a similar divergence.26 Outside of this CSW population, no seroprevalence data exist on trends in HIV prevalence in Senegal since 1996. Moreover, changes in the prevalence of dual HIV-1/HIV-2 infection have not been well studied in Senegal, although data from other West African countries suggest a stable or increasing trend in prevalence.22,23
We investigated trends in HIV-1, -2 and dual HIV-1/HIV-2 infection prevalences in Senegal using screening data from seven (completed and ongoing) research studies conducted between 1990 and 2009 among women presenting to outpatient clinics in and around Dakar. These data were collected for research purposes other than to track changes in incidence or prevalence of HIV-1, -2 and dual infection, and therefore the characteristics of the screened population differed between studies and within several studies. Due to these limitations, we estimated the relative prevalences of HIV-1, -2 and dual infection in order to make unbiased comparisons over the 20-year period. Changes in the relative prevalences were interpreted using the prevalence data of a subset of subjects that were screened for HIV in an unbiased way.
METHODS
Study population
To study trends in HIV prevalence in Senegal, we combined HIV screening data from seven studies conducted in and around Dakar from 1990 to 2009. Subjects were recruited from the outpatient populations of eight clinics, which we will refer to in an abbreviated fashion and which are summarized in Table 1.
Table 1.
Outpatient study clinics and descriptions
| Abbreviated clinic name | Description | Location |
|---|---|---|
| ASBEF | ASBEF family planning clinic | Dakar |
| Dantec | Dantec Hospital oncology clinic | Dakar |
| Fann | University of Dakar Fann outpatient infectious disease clinic | Dakar |
| IHS | A sexually transmitted infection (STI) clinic (Institut d’Hygiene Sociale) | Dakar |
| Mbour | An STI clinic | Mbour (~62 km south of Dakar) |
| Pikine | A primary care clinic | Pikine (~25 km east of Dakar) |
| Sebik | An STI clinic | Sebikotane (~54 km east of Dakar) |
| Thies | An STI clinic | Thies (~58 km east of Dakar) |
These populations have been previously described in the literature, although the sample size of the populations included in the present study differs from those described in relevant publications due to missing HIV screening data for some subjects. All but one study included only women. To avoid bias, in the present analysis we have only included studies in which the participants were screened without regard to suspected or known HIV infection and/or type, and further, as data in men were limited to a single five-year study, we have limited the current analysis to women only. The screened populations included in the analysis are listed (Table 2) and any previous publications describing them are referenced.
Table 2.
Parent studies included in the current analysis
| No. | Study title (reference) | Screening years | Study population | Screening site |
|---|---|---|---|---|
| 1 | Cervical neoplasia and HIV infection in Senegal27 | 1990–1993 | 1025 female CSWs | STI clinics in Dakar (IHS), Thies and Mbour |
| 2 | Natural history of cervical neoplasia in HIV-1 and HIV-228,29 | 1994–1997 | 5392 female CSWs, adult infectious disease clinic patients, female patients of a family planning clinic | STI clinics in Dakar (IHS) and Mbour; the Fann infectious disease clinic and ASBEF family planning clinic |
| 3 | Epidemiology of HIV-1 and HIV-2 oral disease30* | 1994–1997 | 4119 female infectious disease clinic patients | The Fann infectious disease clinic |
| 4 | Epidemiology of oral HIV infection in high-risk women31 | 2000–2004 | 940 female CSWs | STI clinics in Dakar (IHS), Mbour and Sebikotane |
| 5 | Developing new approaches for cervical cancer control32 | 2003–2006 | 884 female patients | Fann infectious disease clinic, Dantec oncology clinic |
| 6 | HIV-associated DNA hypermethylation in cervical cancer | 2006–2010 | 1639 female patients | Fann infectious disease clinic, Dantec oncology clinic, Pikine primary care clinic |
| 7 | Chlamydia and aberrant DNA methylation in cervical cancer | 2007–2011 | 500 female patients | Fann infectious disease clinic, Dantec oncology clinic and Pikine primary care clinic |
STI = sexually transmitted infection
All female subjects in this study were also enrolled in study number 2 (Natural History of Cervical Neoplasia in HIV-1 and HIV-2). Therefore, the numbers of subjects in each study population do not sum to the total number of subjects. The earliest screening year of subjects enrolled in both studies was used for analysis
Data collection
Procedures for collection of subject demographic, medical and sexual behaviour data27–29,31,33,34 and blood sample testing27,31,34 have been previously described. Briefly, subjects completed a standardized interview and blood samples were collected and tested for antibody to HIV-1 and HIV-2, either using an enzyme-linked immunosorbent assay (ELISA; Genetic Systems, Seattle, WA, USA) or a microwell plate enzyme immunoassay (EIA: HIV 1/2 EIA; Sanofi Diagnostics Pasteur, Paris, France). For the subjects screened in the first study and some of the subjects screened in the second (see ref.28 for a description of these subjects), positive results were confirmed with a Western blot (Genetic Systems) and for all studies HIV-1 and HIV-2 were distinguished by a peptide-based assay, although the specific assay used varied by study (Genie II, Genetic Systems; Multispot, Sanofi Diagnostics Pasteur; Immunocomb II, bispot, Orgenics, Yavne, Israel). Dual HIV-1/HIV-2 infection was defined as confirmed dual seropositivity to HIV-1 and HIV-2 by the testing procedures described above.
Statistical methods
For each year from 1990 to 2009, the relative prevalences of HIV-1, -2 and dual HIV-1/HIV-2 infection were calculated for all subjects who were screened. Relative, rather than absolute, prevalence was calculated in order to make unbiased comparisons across studies with different screened populations. Binary logistic regression was used to describe the trends in the relative prevalences of HIV-1. Because the type-specific relative prevalences of the three HIV infection types are not independent (since they must sum to 100%), we used multinomial regression to describe the trends in the relative prevalences of HIV-2 and dual infection as compared with the relative prevalence of HIV-1 infection. For both the binary and multinomial logistic regression analyses, all possible one-knot spline models were fit to describe trends in relative prevalence. The model having the largest likelihood ratio statistic was chosen as the best fit for the relative prevalence of HIV-1 and for the relative prevalence of HIV-2, and dual infection relative to that of HIV-1. Age and birthplace outside of Senegal were considered as a priori confounders in adjusted models. Commercial sex work was not considered as a possible confounding variable because both CSWs and non-CSWs were screened over only six years of the 20-year study, thus preventing adequate adjustment for this variable. To interpret changes in the relative prevalence of HIV from 1990 to 2009, the absolute prevalences of HIV-1, -2 and dual infection were calculated for the Dakar IHS STI clinic, a site where there was no change in the screened population over time. All possible binary logistic regression spline models were fit to describe trends in absolute prevalences and a best fit model was chosen for the prevalence of each HIV infection type. We used t-tests to compare proportions. All analyses were two-sided and used a P < 0.05 level of statistical significance. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
A total of 10,312 women were screened for HIV, 942 of whom were found to be infected with HIV-1, 267 with HIV-2 and 107 with dual HIV-1/HIV-2 infection (Table 3). HIV prevalence varied greatly by screening site and was low among asymptomatic subjects presenting to general health clinics (Pikine 0.4% and ASBEF 1.7%), and higher among symptomatic subjects presenting to outpatient hospital clinics (Dantec 5.9% and Fann 15.9%) and among CSWs screened at four public health clinics (12.5–19.4%). Among subjects who were HIV-positive, HIV-2-infected subjects were older relative to those with HIV-1 infection (mean ages of 37.5 and 34.4 years, respectively; P < 0.001). HIV-1-infected subjects were less likely to be CSWs relative to subjects with either HIV-2 or dual infection (33.8% of HIV-1-infected subjects versus 53.6% of HIV-2 and 55.1% of dually infected subjects; P < 0.001 for both the comparison of HIV-1 with HIV-2, and for the comparison of HIV-1 with dual infection) and these associations persisted even after adjustment for age. There were no other significant differences by HIV type with regard to subject age, country of birth or commercial sex work.
Table 3.
Demographic characteristics of women who were screened for HIV at Senegal clinics, 1990–2009
| Overall (n = 10312)*
|
Seronegative (n = 8996)*
|
HIV-1 (n = 942)*
|
HIV-2 (n = 267)*
|
HIV-D (n = 107)*
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Screening site | ||||||||||
| Fann | 4871 | (47.2) | 4097 | (45.5) | 614 | (65.2) | 115 | (43.1) | 45 | (42.1) |
| Dantec | 692 | (6.7) | 651 | (7.2) | 25 | (2.7) | 12 | (4.5) | 4 | (3.7) |
| Pikine | 1511 | (14.7) | 1505 | (16.7) | 4 | (0.4) | 2 | (0.8) | 0 | (0.0) |
| ASBEF | 230 | (2.2) | 226 | (2.5) | 3 | (0.3) | 0 | (0.0) | 1 | (0.9) |
| Mbour | 758 | (7.4) | 611 | (6.8) | 83 | (8.8) | 49 | (18.4) | 15 | (14.0) |
| IHS | 2050 | (19.9) | 1734 | (19.3) | 196 | (20.8) | 79 | (29.6) | 41 | (38.3) |
| Sebik | 112 | (1.1) | 95 | (1.1) | 15 | (1.6) | 1 | (0.4) | 1 | (0.9) |
| Thies | 88 | (0.9) | 77 | (0.9) | 2 | (0.2) | 9 | (3.4) | 0 | (0.0) |
| Age (years) | ||||||||||
| Under 30 | 3397 | (33.1) | 3003 | (33.5) | 312 | (33.2) | 53 | (19.9) | 29 | (27.6) |
| 30–39 | 3491 | (34.0) | 2991 | (33.4) | 353 | (37.6) | 109 | (40.8) | 38 | (36.2) |
| 40–49 | 2231 | (21.7) | 1917 | (21.4) | 214 | (22.8) | 70 | (26.2) | 30 | (28.6) |
| 50+ | 1152 | (11.2) | 1049 | (11.7) | 60 | (6.4) | 35 | (13.1) | 8 | (7.6) |
| Ethnicity | ||||||||||
| Wolof | 3796 | (44.6) | 3357 | (46.4) | 308 | (34.0) | 97 | (37.2) | 34 | (33.7) |
| Pular | 1707 | (20.1) | 1382 | (19.1) | 247 | (27.2) | 63 | (24.1) | 15 | (14.9) |
| Serere | 1051 | (12.4) | 898 | (12.4) | 101 | (11.1) | 36 | (13.8) | 16 | (15.8) |
| Other | 1949 | (22.9) | 1597 | (22.1) | 251 | (27.7) | 65 | (24.9) | 36 | (35.6) |
| Birthplace | ||||||||||
| Senegal | 8892 | (90.1) | 7896 | (91.1) | 704 | (82.6) | 214 | (86.3) | 78 | (78.0) |
| Ghana | 401 | (4.1) | 331 | (3.8) | 58 | (6.8) | 3 | (1.2) | 9 | (9.0) |
| Other country in West Africa | 512 | (5.2) | 391 | (4.5) | 79 | (9.3) | 29 | (11.7) | 13 | (13.0) |
| Other | 67 | (0.7) | 54 | (0.6) | 11 | (1.3) | 2 | (0.8) | 0 | (0.0) |
| Smoking | ||||||||||
| No | 8106 | (81.9) | 7177 | (82.5) | 682 | (80.1) | 168 | (67.7) | 79 | (79.0) |
| Yes | 1796 | (18.1) | 1526 | (17.5) | 169 | (19.9) | 80 | (32.3) | 21 | (21.0) |
| Alcohol use | ||||||||||
| No | 8780 | (88.8) | 7793 | (89.7) | 713 | (84.1) | 192 | (77.4) | 82 | (82.0) |
| Yes | 1109 | (11.2) | 900 | (10.4) | 135 | (15.9) | 56 | (22.6) | 18 | (18.0) |
| Marital status | ||||||||||
| Married (monogamous) | 1338 | (13.2) | 1184 | (13.4) | 118 | (12.7) | 24 | (9.1) | 12 | (11.7) |
| Married (polygamous) | 3336 | (32.8) | 3038 | (34.3) | 229 | (24.7) | 50 | (18.9) | 19 | (18.5) |
| Widow | 2415 | (23.8) | 2231 | (25.2) | 128 | (13.8) | 43 | (16.3) | 13 | (12.6) |
| Divorced | 1444 | (14.21) | 1194 | (13.5) | 146 | (15.7) | 81 | (30.7) | 23 | (22.3) |
| Cohabiting | 941 | (9.3) | 755 | (8.5) | 136 | (14.6) | 34 | (12.9) | 16 | (15.5) |
| Single | 680 | (6.7) | 457 | (5.2) | 171 | (18.4) | 32 | (12.1) | 20 | (19.4) |
| Separated | 7 | (0.1) | 6 | (0.1) | 1 | (0.1) | 0 | (0.0) | 0 | (0.0) |
| Contraception | ||||||||||
| None | 5748 | (59.4) | 4944 | (58.3) | 616 | (71.2) | 136 | (56.0) | 52 | (55.3) |
| Pills | 1277 | (13.2) | 1047 | (12.4) | 127 | (14.7) | 71 | (29.2) | 32 | (34.0) |
| Injections | 1166 | (12.1) | 1061 | (12.5) | 71 | (8.2) | 27 | (11.1) | 7 | (7.5) |
| Condoms | 405 | (4.2) | 376 | (4.4) | 26 | (3.0) | 3 | (1.2) | 0 | (0.0) |
| IUD | 351 | (3.6) | 344 | (4.1) | 7 | (0.8) | 0 | (0.0) | 0 | (0.0) |
| Other | 731 | (7.6) | 704 | (8.3) | 18 | (2.1) | 6 | 2.47 | 3 | (3.2) |
| Commercial sex worker | ||||||||||
| No | 7270 | (70.5) | 6474 | (72.0) | 624 | (66.2) | 124 | (46.4) | 48 | (44.9) |
| Yes | 3042 | (29.5) | 2522 | (28.0) | 318 | (33.8) | 143 | (53.6) | 59 | (55.1) |
IUD = intrauterine device
Subtotals may not sum to total n after exclusion of subjects with missing data
Because these prevalence data were not collected from a single population over time, it was not possible to distinguish a true change in prevalence from one due to a change in the underlying risk of HIV infection among the screened individuals. We therefore focused our analysis on the investigation of trends in the relative prevalences of HIV-1, -2 and dual infection (Table 4, Figure 1).
Table 4.
Relative prevalence of HIV among women presenting to Senegal clinics, 1990–2009
| Screening year | Number seropositive | HIV-1
|
HIV-2
|
HIV-D
|
|||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| 1990 | 48 | 18 | (37.5) | 26 | (54.2) | 4 | (8.3) |
| 1991 | 62 | 24 | (38.7) | 28 | (45.2) | 10 | (16.1) |
| 1992 | 48 | 31 | (64.6) | 12 | (25.0) | 5 | (10.4) |
| 1993 | 4 | 2 | (50.0) | 2 | (50.0) | 0 | (0.0) |
| 1994 | 50 | 34 | (68.0) | 8 | (16.0) | 8 | (16.0) |
| 1995 | 221 | 150 | (67.9) | 50 | (22.6) | 21 | (9.5) |
| 1996 | 174 | 129 | (74.1) | 30 | (17.2) | 15 | (8.6) |
| 1997 | 154 | 126 | (81.8) | 18 | (11.7) | 10 | (6.5) |
| 1998 | No HIV screening performed | ||||||
| 1999 | No HIV screening performed | ||||||
| 2000 | 5 | 4 | (80.0) | 0 | (0.0) | 1 | (20.0) |
| 2001 | 74 | 48 | (64.9) | 20 | (27.0) | 6 | (8.1) |
| 2002 | 41 | 30 | (73.2) | 6 | (14.6) | 5 | (12.2) |
| 2003 | 60 | 46 | (76.7) | 11 | (18.3) | 3 | (5.0) |
| 2004 | 77 | 55 | (71.4) | 15 | (19.5) | 7 | (9.1) |
| 2005 | 51 | 42 | (82.4) | 8 | (15.7) | 1 | (2.0) |
| 2006 | 85 | 75 | (88.2) | 9 | (10.6) | 1 | (1.2) |
| 2007 | 14 | 11 | (78.6) | 2 | (14.3) | 1 | (7.1) |
| 2008 | 105 | 86 | (81.9) | 13 | (12.4) | 6 | (5.7) |
| 2009 | 43 | 31 | (72.1) | 9 | (20.9) | 3 | (7.0) |
| Total | 1316 | 942 | (71.6) | 267 | (20.3) | 107 | (8.1) |
HIV-D = HIV-1/HIV-2 dual infection
Figure 1.
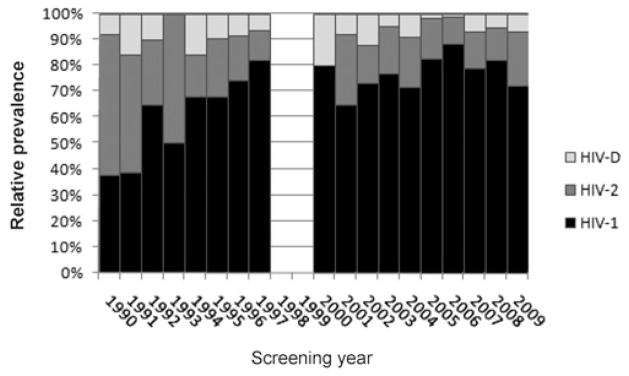
Trends in the relative prevalence of HIV-1, -2 and dual HIV-1/HIV-2 infection in women in Senegal from 1990 to 2009. HIV-D indicates HIV-1/HIV-2 dual infection
Because the relative prevalences of HIV-1 and HIV-2 appeared to change during the 1990s and stabilize thereafter (Table 4, PFigure 1), we sought to identify a year marking a change from a period of trend to a period of stabilization in the relative prevalences of HIV-1 and HIV-2 infections. We also sought to confirm our subjective assessment that there was little change in the relative prevalence of dual HIV infection over the time period. To this end, we found the best fit binary logistic regression model for the odds of infection with HIV-1 and best fit multinomial logistic regression model for the odds of all three HIV infection types, allowing for two periods of differing slope. For HIV-1, dividing the screening years into two periods at 1993 resulted in the best fit adjusted model; from 1990 to 1993, the relative prevalence of HIV-1 increased markedly (odds ratio [OR] associated with a 1-year change in time = 1.71, < 0.001) and continued to increase, albeit at a slower rate (OR = 1.08, P < 0.001), through 2009. In the multinomial logistic model of the relative prevalences, dividing the screening years into two periods at 1993 likewise yielded the best fit adjusted model. During the period from 1990 to 1993, the relative odds of HIV-2 infection decreased relative to that of HIV-1 (OR = 0.54; P < 0.001), and continued to decrease slightly from 1993 to 2009 (OR = 0.93; P < 0.001). The relative odds of dual infection was stable as compared with the relative odds of HIV-1 infection over the period from 1990 to 1993 (OR = 0.80; P = 0.14) but decreased thereafter (OR = 0.91; P < 0.001).
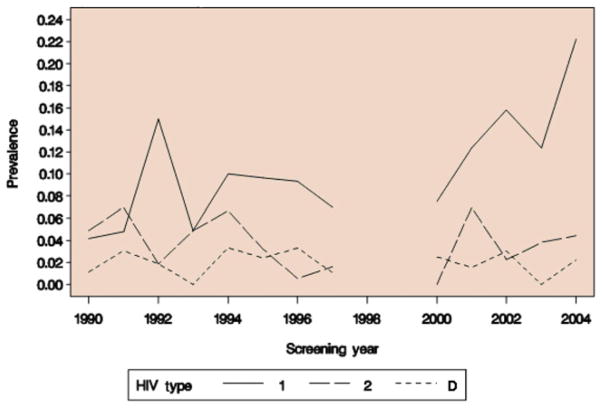
To confirm that the overall trends we observed in the relative prevalence of the three HIV infection types were not due to mixing of various low- and high-risk study populations over time, we examined the trends in prevalences of subjects screened at a single clinic, the IHS STI clinic, where the screened population (CSWs) remained constant over time (Figure 2). No subjects were screened at this clinic from 1998 to 1999 or after 2004. Therefore, the trends in the prevalences of HIV-1, -2 and dual infection were examined over the periods from 1990 to 1997, from 2000 to 2004 and from 1990 to 2004. Using binary logistic regression, we found that the odds of HIV-1 infection increased from 1990 to 1997 and from 2000 to 2004, but neither of these trends reached statistical significance (OR = 1.07, P = 0.08 from 1990 to 1997; OR = 1.20, P = 0.10 from 2000 to 2004). A significant increase in the odds of HIV-1 infection was, however, observed over the entire period from 1990 to 2004 (OR = 1.08; P < 0.001). The odds of HIV-2 infection decreased significantly from 1990 to 1997 (OR = 0.83; P = 0.002), but there was no significant trend from 2000 to 2004 (OR = 0.89; P = 0.54). Over the entire period from 1990 to 2004, there was also no trend in the odds of HIV-2 infection (OR = 0.99; P = 0.57). The likelihood of dual infection did not change significantly over any of the time periods (all P values >0.65).
Figure 2.
Trends in the absolute prevalence of HIV-1, -2 and dual HIV-1/HIV-2 infection in Dakar (IHS) sexually transmitted infection clinic outpatients from 1990 to 2004. HIV type D denotes HIV-1/HIV-2 dual infection
DISCUSSION
During the last 30 years, Senegal has been considered a success story in the fight against HIV/AIDS, as it has experienced a consistently low HIV prevalence (less than 1.5%) in the general population throughout the epidemic, although HIV prevalence among CSWs and men who have sex with men throughout Senegal is greater than 15%.26,31,35,36 A number of factors including political and social stability, the country’s conservative sexual norms, early prevention efforts by the public health sector as well as non-governmental agencies (STI screening and treatment and condom provisions among CSWs, screening of the blood supply by 1987) and geography (Senegal is on the westernmost tip of Africa) most likely have all played a role in the containment of HIV to high-risk core groups such as CSWs. The presence of HIV-2 prior to the introduction of HIV-1 into the population may also have been a contributing factor.
We aimed to investigate trends in the relative prevalences of HIV-1, -2 and dual infections in Senegal by analysing the screening data of 10,321 female outpatients attending clinics in and around Dakar between 1990 and 2009. In these outpatient populations, HIV prevalence was low (<2%) in asymptomatic subjects screened at general outpatients clinics and was greater in symptomatic subjects presenting to hospital-based clinics for care (6–20%) and among CSWs (12–20%). Women infected with HIV-2 were older than those infected with HIV-1. A greater prevalence of HIV-2 infection in older age groups has been observed in other populations in West Africa18,19,37 and has been explained by the endemicity of HIV-2 in the region and the slower progression of HIV-2 relative to HIV-1.37 CSWs were more likely to be HIV-2 or dually infected compared with non-CSWs; this association between CSW and HIV infection type has been observed in other populations in Senegal38,39 and may be the result of differences in the transmission dynamics between the two viruses as well as differences in the numbers of sex partners and HIV exposure between CSW and non-CSW populations.8,11,12
Over a 20-year period of HIV screening, we observed a marked increase in the relative prevalence of HIV-1 from 1990 to 1993, followed by a slight increase from 1993 to 2009. The relative prevalence of HIV-2 infection decreased markedly relative to that of HIV-1 through 1993, which was followed by a period of a slight decrease in relative prevalence. The relative prevalence of dual infection was stable as compared with that of HIV-1 from 1990 to 1993 and decreased thereafter. The trends we observed are mostly compatible with mathematical models which have predicted that, because HIV-2 is associated with substantially lower viral loads in the plasma and genital tract, and is thus less efficiently transmitted,8–13 in the long term HIV-1 will always outcompete and competitively displace HIV-2 when both viruses are circulating in the same population.40 However, these models also suggest that it may take decades before HIV-2 is eliminated in a population, during which time HIV-1 would increase, HIV-2 prevalence would be fairly stable and then decrease, and dual infection would slowly increase and then decrease. Because these trends in relative prevalence could be attributable to a change in one, two or all of the HIV infection types, we used prevalence data collected from a subset of our study population, infectious disease clinic patients, to help interpret these trends. The prevalence data from this subset of patients suggest that the changes in HIV-1 and HIV-2 relative prevalences observed during the 1990s (during the 8 years in which HIV screening data were collected) were due to an increase in HIV-1 prevalence and a decrease in HIV-2 and dual infection prevalences during this period. The stability of the relative prevalences of HIV-1, -2 and dual infection in the 2000s are reflective of the stable prevalences observed in the subset of infectious disease clinic patients. Because only women were included in our study population, our findings with regard to trends of HIV-1 and HIV-2 in Senegal may be most generalizable to women. We were also unable to completely control for the possible effect of changes in patient characteristics over time on the trends in the relative prevalences due to limitations of our data. Specifically, we could not adequately adjust for the possible effect of CSW on the relative prevalences of HIV infection types over time because CSWs and non-CSWs were simultaneously screened over a period of only six years and restriction to either CSWs or non-CSWs would have resulted in the exclusion of five or seven years of data, respectively. Given that the rates of transmission of HIV-1 and HIV-2 infections between CSWs and the other subject populations over this time period are unknown, it is unclear to what extent this limitation may have biased our results. Additionally, during the course of these studies, a number of HIV peptide-based assays to categorize HIV-1 and HIV-2 seropositivity were utilized and the specific assay used varied by study. If these assays differed in their ability to distinguish HIV-1 from HIV-2, this may have biased our findings; however, given the high HIV-1 versus HIV-2 specificity of all assays used, assay differences are unlikely to have resulted in substantial misclassification of subjects.
Regardless of these shortcomings, this study does have a number of strengths: most notably its sample size, which, to our knowledge, is the largest of any HIV prevalence study conducted in Senegal. This study is also the first to present HIV screening data collected more recently than 2004 and the first to present screening data from a female population outside of CSWs since 1996. We were also able to confirm the results of other studies that demonstrated diverging trends in HIV-1 and HIV-2 prevalence in West Africa during the 1990s. This study provides further evidence of a stabilization of the prevalence of HIV infection types since 2000, which was previously demonstrated in Senegal.25 Future research is needed to confirm recent trends in prevalence in other populations in Senegal.
Despite the decreasing trends we observed in the relative prevalence of HIV-2 infection, HIV-2 remains endemic in West Africa and 15–30% of HIV seropositive women in Senegal are infected with HIV-2 or HIV-1/HIV-2 dual infection. However, to date there has not been a single randomized clinical trial of antiretroviral therapy (ART) for HIV-2 infection. Given that HIV-2- and dual HIV-1/HIV-2-infected individuals respond poorly to first-line ART,41,42 the persistence of HIV-2 and dual infection observed in this study underlines the importance identifying HIV infection type before initiating ART in a Senegalese population and establishing evidence-based ART guidelines in HIV-2- and dually-infected individuals.
Acknowledgments
We would like to thank the Dakar area outpatient health clinics that supported this study and all study participants. We would also like to thank Papa Toure, Birama Dembele, Charlotte Faty Ndiaye, Awa Gaye, Elizabeth Benga, Awa Coll-Seck, Mame Awa Faye-Niang, Charlotte Sarr, Mame Dieumbe Mbengue, Abdoul Aziz Kasse, Assatou Diop, Amadou Dem and Cathy Critchlow for their leadership of studies and/or clinics that contributed data to this analysis; Mame Birame Diouf, Elise Reay-Ellers and Deana Rich for their study coordination in Senegal; Haby Diallo-Agne, Diouana Ba, Papa Ousmange Diop, Maguette Diongue, Sophie Chablis, Jane Kuyppers, Natalie Zheng, Steve Cherne, Donna Kenney, Fatou Traore and Qinghua Feng for laboratory processing, analysis and supervision; and Alison Starling, John Lin, Joshua Stern, Fatou Faye-Diop and Fatima Sall for forms development and data management. This study was supported by NIH grants CA50856, CA62801, DE11372, DE12925, AI48470, CA097275, CA111187 and CA115713.
References
- 1.UNAIDS. AIDS Epidemic Update. Geneva: UNAIDS; 2010. [Google Scholar]
- 2.Fultz PN, Switzer WM, Schable CA, Desrosiers RC, Silva DP, McCormick JB. Seroprevalence of HIV-1 and HIV-2 in Guinea Bissau in 1980. AIDS. 1988;2:129–32. doi: 10.1097/00002030-198804000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Fleming AF. Seroepidemiology of human immunodeficiency viruses in Africa. Biomed Pharmacother. 1988;42:309–20. [PubMed] [Google Scholar]
- 4.Kawamura M, Yamazaki S, Ishikawa K, Kwofie TB, Tsujimoto H, Hayami M. HIV-2 in west Africa in 1966. Lancet. 1989;1:385. doi: 10.1016/s0140-6736(89)91760-1. [DOI] [PubMed] [Google Scholar]
- 5.Lemey P, Pybus OG, Wang B, Saksena NK, Salemi M, Vandamme AM. Tracing the origin and history of the HIV-2 epidemic. Proc Natl Acad Sci USA. 2003;100:6588–92. doi: 10.1073/pnas.0936469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marlink R, Kanki P, Thior I, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265:1587–90. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 7.Norrgren H, da Silva Z, Biague A, Andersson S, Biberfeld G. Clinical progression in early and late stages of disease in a cohort of individuals infected with human immunodeficiency virus-2 in Guinea-Bissau. Scand J Infect Dis. 2003;35:265–72. doi: 10.1080/00365540310000210. [DOI] [PubMed] [Google Scholar]
- 8.Kanki PJ, Travers KU, Boup MS, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343:943–6. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly C, Leisenring W, Kanki P, Awerbuch T, Sandberg S. Comparison of transmission rates of HIV-1 and HIV-2 in a cohort of prostitutes in Senegal. Bull Math Biol. 1993;55:731–43. doi: 10.1007/BF02460671. [DOI] [PubMed] [Google Scholar]
- 10.O’Donovan D, Ariyoshi K, Milligan P, et al. Maternal plasma viral RNA levels determine marked differences in mother-to-child transmission rates of HIV-1 and HIV-2 in The Gambia. MRC/Gambia Government/University College London Medical School working group on mother-child transmission of HIV. AIDS. 2000;14:441–8. doi: 10.1097/00002030-200003100-00019. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb GS, Hawes SE, Agne HD, et al. Lower levels of HIV RNA in semen in HIV-2 compared with HIV-1 infection: implications for differences in transmission. AIDS. 2006;20:895–900. doi: 10.1097/01.aids.0000218554.59531.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popper SJ, Sarr AD, Travers KU, et al. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis. 1999;180:1116–21. doi: 10.1086/315010. [DOI] [PubMed] [Google Scholar]
- 13.Hawes SE, Sow PS, Stern JE, Critchlow CW, Gottlieb GS, Kiviat NB. Lower levels of HIV-2 than HIV-1 in the female genital tract: correlates and longitudinal assessment of viral shedding. AIDS. 2008;22:2517–25. doi: 10.1097/QAD.0b013e328315cdbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nkengasong JN, Kestens L, Ghys PD, et al. Dual infection with human immunodeficiency virus type 1 and type 2: impact on HIV type 1 viral load and immune activation markers in HIV-seropositive female sex workers in Abidjan, Ivory Coast. AIDS Res Hum Retroviruses. 2000;16:1371–8. doi: 10.1089/08892220050140919. [DOI] [PubMed] [Google Scholar]
- 15.Alabi AS, Jaffar S, Ariyoshi K, et al. Plasma viral load, CD4 cell percentage, HLA and survival of HIV-1, HIV-2, and dually infected Gambian patients. AIDS. 2003;17:1513–20. doi: 10.1097/00002030-200307040-00012. [DOI] [PubMed] [Google Scholar]
- 16.Schim van der Loeff MF, Jaffar S, Aveika AA, et al. Mortality of HIV-1, HIV-2 and HIV-1/HIV-2 dually infected patients in a clinic-based cohort in The Gambia. AIDS. 2002;16:1775–83. doi: 10.1097/00002030-200209060-00010. [DOI] [PubMed] [Google Scholar]
- 17.van Tienen C, Schim van der Loeff M, Peterson I, et al. HTLV-1 and HIV-2 infection are associated with increased mortality in a rural West African community. PLoS One. 2011;6:e29026. doi: 10.1371/journal.pone.0029026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Månsson F, Biague A, da Silva ZJ, et al. Prevalence and incidence of HIV-1 and HIV-2 before, during and after a civil war in an occupational cohort in Guinea-Bissau, West Africa. AIDS. 2009;23:1575–82. doi: 10.1097/QAD.0b013e32832cedfb. [DOI] [PubMed] [Google Scholar]
- 19.Månsson F, Alves A, Silva ZJ, et al. Trends of HIV-1 and HIV-2 prevalence among pregnant women in Guinea-Bissau, West Africa: possible effect of the civil war 1998–1999. Sex Transm Infect. 2007;83:463–7. doi: 10.1136/sti.2007.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tienen C, van der Loeff MS, Zaman SM, et al. Two distinct epidemics: the rise of HIV-1 and decline of HIV-2 infection between 1990 and 2007 in rural Guinea-Bissau. J Acquir Immune Defic Syndr. 2010;53:640–7. doi: 10.1097/QAI.0b013e3181bf1a25. [DOI] [PubMed] [Google Scholar]
- 21.da Silva ZJ, Oliveira I, Andersen A, et al. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS. 2008;22:1195–202. doi: 10.1097/QAD.0b013e328300a33d. [DOI] [PubMed] [Google Scholar]
- 22.Djomand G, Greenberg AE, Sassan-Morokro M, et al. The epidemic of HIV/AIDS in Abidjan, Côte d’Ivoire: a review of data collected by Projet RETRO-CI from 1987 to 1993. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:358–65. [PubMed] [Google Scholar]
- 23.van der Loeff MF, Awasana AA, Sarge-Njie R, et al. Sixteen years of HIV surveillance in a West African research clinic reveals divergent epidemic trends of HIV-1 and HIV-2. Int J Epidemiol. 2006;35:1322–8. doi: 10.1093/ije/dyl037. [DOI] [PubMed] [Google Scholar]
- 24.Schim van der Loeff MF, Sarge-Njie R, Ceesay S, et al. Regional differences in HIV trends in The Gambia: results from sentinel surveillance among pregnant women. AIDS. 2003;17:1841–6. doi: 10.1097/01.aids.0000076303.76477.49. [DOI] [PubMed] [Google Scholar]
- 25.Hamel DJ, Sankalé JL, Eisen G, et al. Twenty years of prospective molecular epidemiology in Senegal: changes in HIV diversity. AIDS Res Hum Retroviruses. 2007;23:1189–96. doi: 10.1089/aid.2007.0037. [DOI] [PubMed] [Google Scholar]
- 26.Meda N, Ndoye I, M’Boup S, et al. Low and stable HIV infection rates in Senegal: natural course of the epidemic or evidence for success of prevention? AIDS. 1999;13:1397–405. doi: 10.1097/00002030-199907300-00018. [DOI] [PubMed] [Google Scholar]
- 27.Langley CL, Benga-De E, Critchlow CW, et al. HIV-1, HIV-2, human papillomavirus infection and cervical neoplasia in high-risk African women. AIDS. 1996;10:413–7. doi: 10.1097/00002030-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Ali S, Niang MA, N’doye I, et al. Secretor polymorphism and human immunodeficiency virus infection in Senegalese women. J Infect Dis. 2000;181:737–9. doi: 10.1086/315234. [DOI] [PubMed] [Google Scholar]
- 29.Hawes SE, Critchlow CW, Faye Niang MA, et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis. 2003;188:555–63. doi: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- 30.Pavlinac PB, Hawes SE, Gottlieb GS, et al. HIV shedding in the oral cavity: an assessment of HIV type, immunovirologic, demographic and oral factors. Sex Transm Infect. 2012;88:45–50. doi: 10.1136/sextrans-2011-050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Hawes SE, Gaye A, et al. HIV prevalence, previous HIV testing, and condom use with clients and regular partners among Senegalese commercial sex workers. Sex Transm Infect. 2007;83:534–40. doi: 10.1136/sti.2007.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Q, Balasubramanian A, Hawes SE, et al. Detection of hypermethylated genes in women with and without cervical neoplasia. J Natl Cancer Inst. 2005;97:273–82. doi: 10.1093/jnci/dji041. [DOI] [PubMed] [Google Scholar]
- 33.Ndiaye CF, Critchlow CW, Leggott PJ, et al. Periodontal status of HIV-1 and HIV-2 seropositive and HIV seronegative female commercial sex workers in Senegal. J Periodontol. 1997;68:827–31. doi: 10.1902/jop.1997.68.9.827. [DOI] [PubMed] [Google Scholar]
- 34.Zheng NN, Kiviat NB, Sow PS, et al. Comparison of human immunodeficiency virus (HIV)-specific T-cell responses in HIV-1- and HIV-2-infected individuals in Senegal. J Virol. 2004;78:13934–42. doi: 10.1128/JVI.78.24.13934-13942.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade AS, Kane CT, Diallo PA, et al. HIV infection and sexually transmitted infections among men who have sex with men in Senegal. AIDS. 2005;19:2133–40. doi: 10.1097/01.aids.0000194128.97640.07. [DOI] [PubMed] [Google Scholar]
- 36.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. UNAIDS; 2010. [Google Scholar]
- 37.Wilkins A, Ricard D, Todd J, Whittle H, Dias F, Paulo Da Silva A. The epidemiology of HIV infection in a rural area of Guinea-Bissau. AIDS. 1993;7:1119–22. doi: 10.1097/00002030-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007;196:887–94. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 39.Hawes SE, Critchlow CW, Sow PS, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst. 2006;98:100–9. doi: 10.1093/jnci/djj010. [DOI] [PubMed] [Google Scholar]
- 40.Anderson RM, May RM. The population biology of the interaction between HIV-1 and HIV-2: coexistence or competitive exclusion? AIDS. 1996;10:1663–73. doi: 10.1097/00002030-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Drylewicz J, Eholie S, Maiga M, et al. First-year lymphocyte T CD4+ response to antiretroviral therapy according to the HIV type in the IeDEA West Africa collaboration. AIDS. 2010;24:1043–50. doi: 10.1097/qad.0b013e3283377a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb GS, Eholié SP, Nkengasong JN, et al. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS. 2008;22:2069–72. doi: 10.1097/QAD.0b013e32830edd44. discussion 73–4 (Accepted 13 May 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]