FIG. 16.
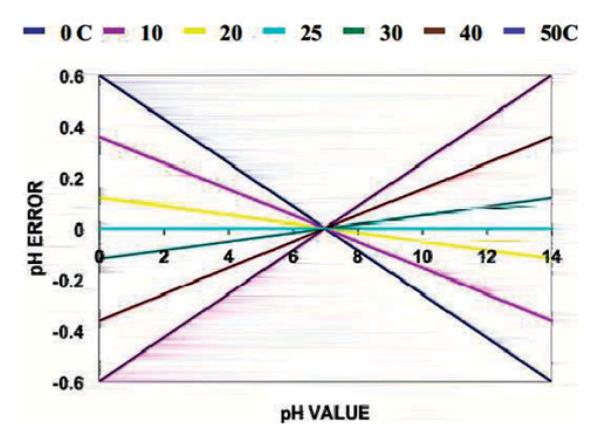
The scope of the temperature-induced pH measurement error for a range of temperatures, as calculated using the Nernst equation: E=Eo—2.3RT(−log[H+])/F, where E is the electric potential measured, Eo is the standard electric potential, R is the gas constant, T is the temperature, and F is the Faraday constant. Reprinted with permission from Queeney.[111] (Figure available in color online.)
