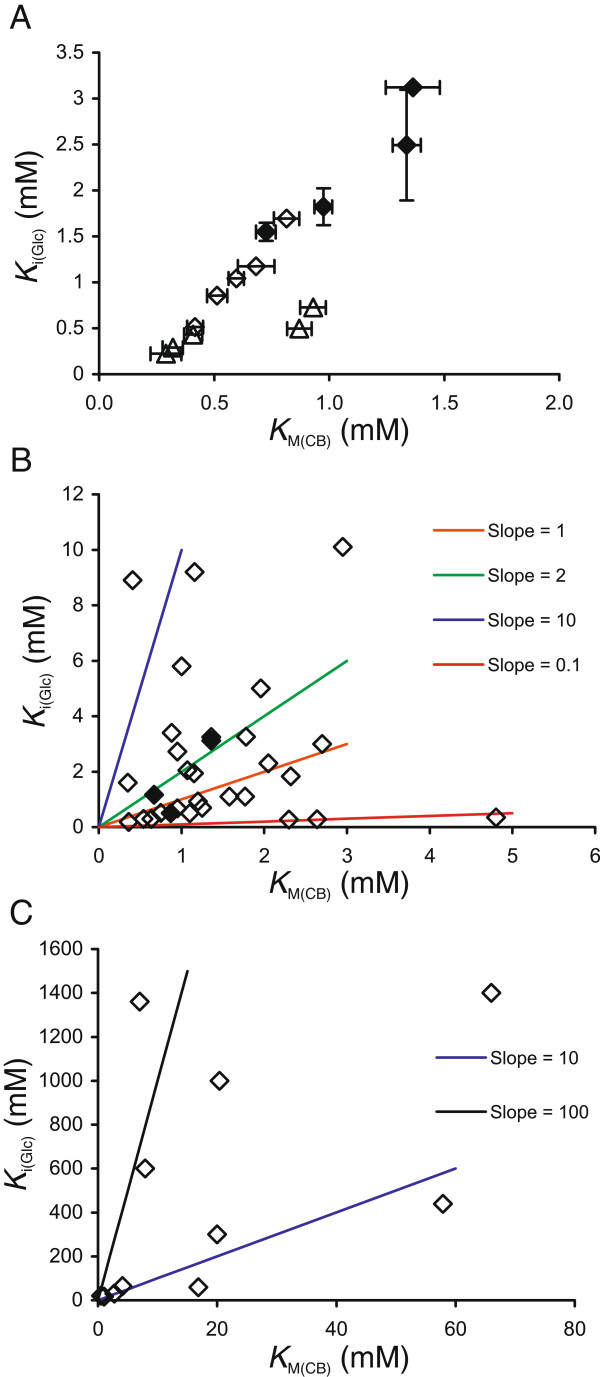
Figure 4.
A higher affinity for cellobiose is accompanied by a stronger glucose inhibition of β-glucosidases (BGs). (A) The values of the Michaelis constants for cellobiose hydrolysis (KM(h)) and the inhibition constants for glucose (Ki(Glc)) are from Table 1 and Table 4, respectively. TaBG3 (◊), AtBG3 (∆) and N188BG (♦). (B and C) A literature survey revealed that BGs can be tentatively divided into three groups based on their relative affinities for cellobiose (KM(CB)) and glucose (Ki(Glc)): (i) KM(CB) > > Ki(Glc), BGs near the red line; (ii) KM(CB) ≈ Ki(Glc), BGs near the pink and the green line and (iii) KM(CB) < <Ki(Glc), BGs near the blue and the black line. For the numerical values of KM(CB) and Ki(Glc), see Table 5. If Ki(Glc) values measured using both pNPG and cellobiose as the substrate were available, the priority was given to the Ki(Glc) value measured using cellobiose. Data from the present study (♦).