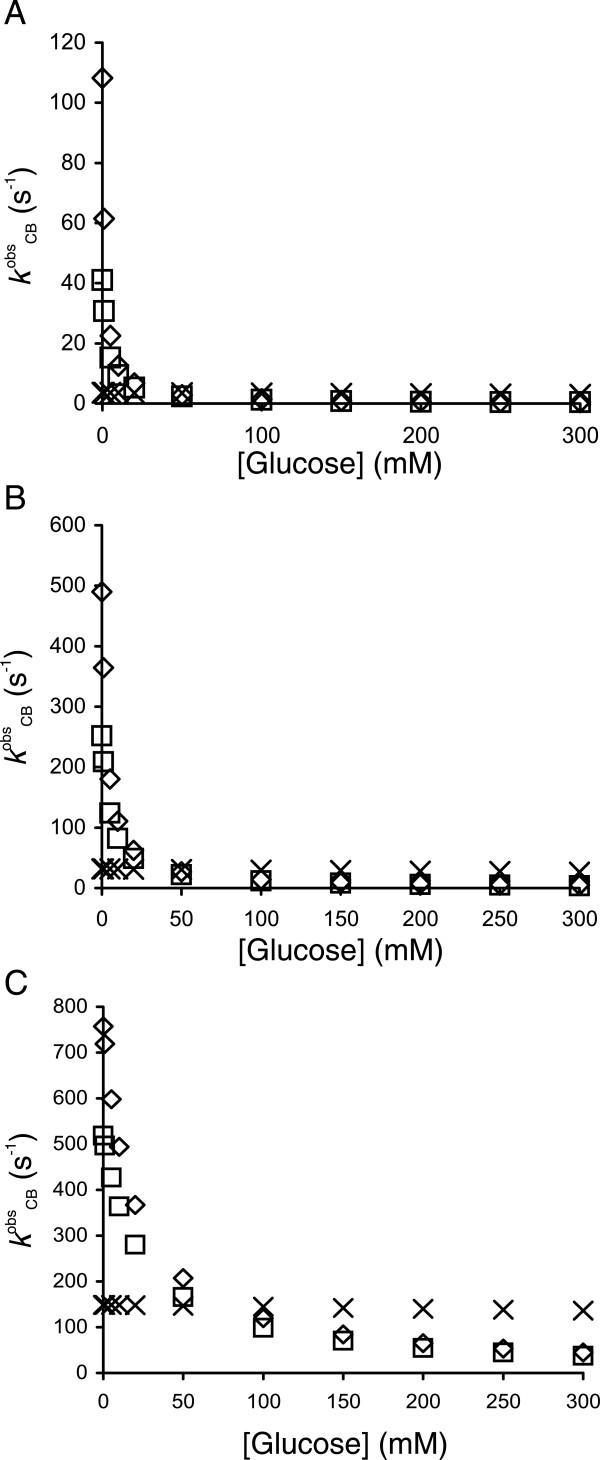
Figure 6.
Calculated values of the rate constants of cellobiose hydrolysis for β-glucosidases with different kinetic properties. The values of the observed rate constants of cellobiose hydrolysis (kobsCB) at different cellobiose and glucose concentrations were calculated using the simple Michaelis-Menten equation with competitive glucose inhibition and ignoring substrate inhibition. The β-glucosidases used were TaBG3 (◊) and N188BG (□), characterized in the present study, and a previously characterized glucose-tolerant β-glucosidase from Aspergillus oryzae (AoBG3) (×) [30]. kcat(h) values of 806 s-1, 587 s-1 and 253 s-1, KM(CB) values of 0.65 mM, 1.33 mM and 7.0 mM and Ki(Glc) values of 1.14 mM, 2.75 mM and 1360 mM were used for TaBG3, N188BG and AoBG3, respectively. The concentration of cellobiose was set to 0.1 mM (A), 1.0 mM (B) or 10 mM (C).