Abstract
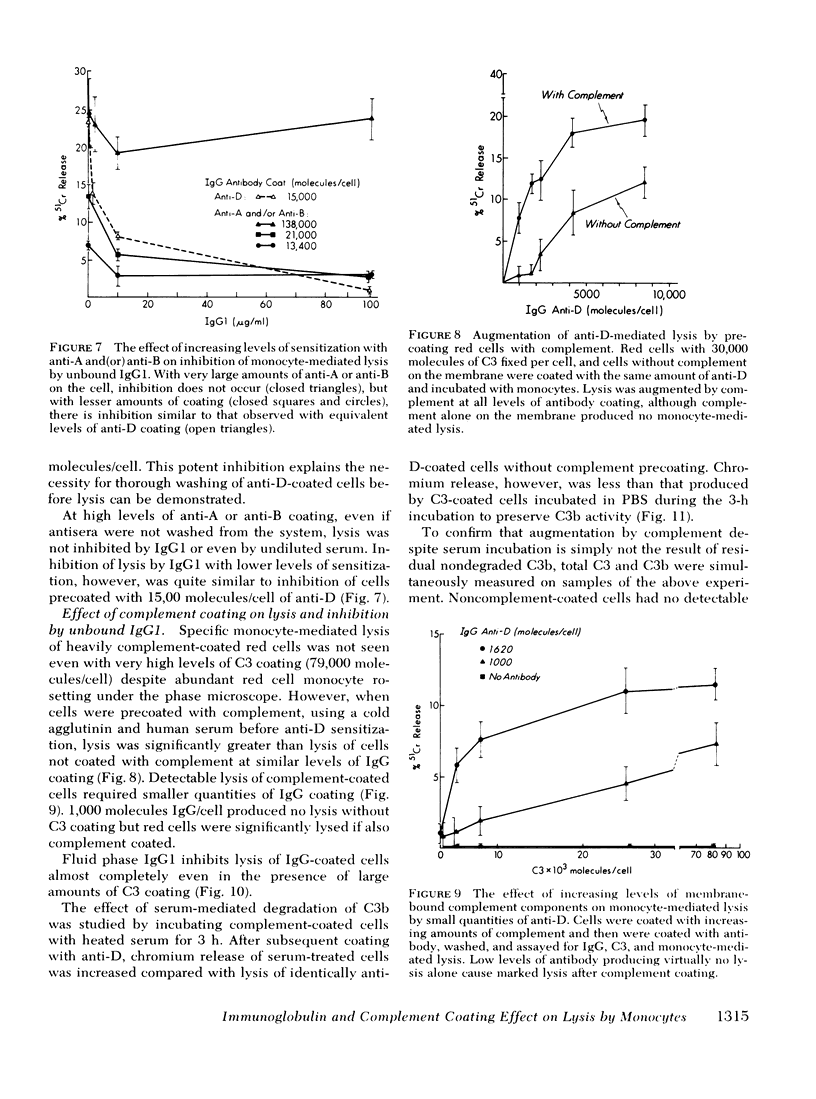
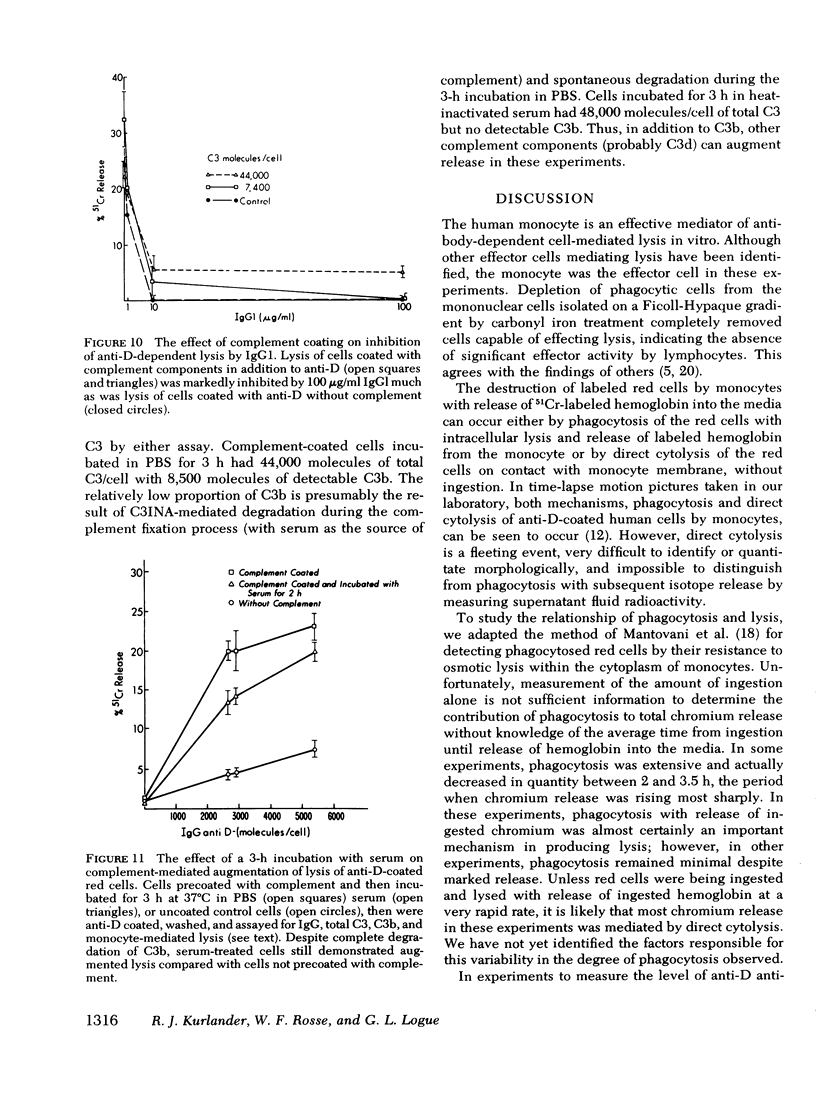
Monocyte-mediated lysis in vitro of human red cells coated with measured amounts of immunoglobulin G (IgG) or complement were studied. 1,000-1,500 molecules of IgG anti-D are necessary to effect measurable lysis, and lysis increases linearly with increasing levels of antibody sensitization. 100 microgram/ml of IgG1 abolished lysis even at maximal levels of anti-D sensitization (15,000 molecules/cell). Two isoimmune IgG anti-A or anti-B antisera were 5 to 10-fold less efficient in promoting phagocytosis or lysis per molecule of IgG bound; however, because of the greater antigen density of A or B, more than 100,000 molecules IgG/cell could be bound, producing equivalent lysis to anti-D-coated cells. Although inhibition by IgG1 was similar at equivalent levels of sensitization with anti-A, anti-B, or anti-D at high levels of coating with anti-A or anti-B (not attainable with anti-D), lysis was not inhibited by IgG1. Cells coated with human complement components alone were not lysed by monocytes; however, complement coating augmented IgG-mediated lysis and reduced the quantity of anti-D necessary to produce lysis to less than 1,000 molecules/cell. After thorough degradation of C3b by serum to C3d, complement augmentation persisted.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Gelfand E. W., Jandl J. H., Rosen F. S. The interaction between human monocytes and red cells. Specificity for IgG subclasses and IgG fragments. J Exp Med. 1970 Dec 1;132(6):1207–1215. doi: 10.1084/jem.132.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. L., Lachmann P. J., Dacie J. V. The in vivo behaviour of complement-coated red cells: studies in C6-deficient, C3-depleted and normal rabbits. Clin Exp Immunol. 1970 Sep;7(3):401–421. [PMC free article] [PubMed] [Google Scholar]
- Gilliland B. C. Coombs--negative immune hemolytic anemia. Semin Hematol. 1976 Oct;13(4):267–275. [PubMed] [Google Scholar]
- Holm G., Engwall E., Hammarström S., Natvig J. B. Antibody-induced hemolytic activity of human blood monocytes. Scand J Immunol. 1974;3(2):173–180. doi: 10.1111/j.1365-3083.1974.tb01245.x. [DOI] [PubMed] [Google Scholar]
- Holm G., Hammarström S. Haemolytic activity of human blood monocytes. Lysis of human erythrocytes treated with anti-A serum. Clin Exp Immunol. 1973 Jan;13(1):29–43. [PMC free article] [PubMed] [Google Scholar]
- Huber H., Douglas S. D., Fudenberg H. H. The IgG receptor: an immunological marker for the characterization of mononuclear cells. Immunology. 1969 Jul;17(1):7–21. [PMC free article] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Logue G., Rosse W. Immunologic mechanisms in autoimmune hemolytic disease. Semin Hematol. 1976 Oct;13(4):277–289. [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplack D. G., Bonnard G. D., Holiman B. J., Blaese R. M. Monocyte-mediated antibody-dependent cellular cytotoxicity: a clinical test of monocyte function. Blood. 1976 Dec;48(6):809–816. [PubMed] [Google Scholar]
- Ross G. D., Polley M. J., Rabellino E. M., Grey H. M. Two different complement receptors on human lymphocytes. One specific for C3b and one specific for C3b inactivator-cleaved C3b. J Exp Med. 1973 Oct 1;138(4):798–811. doi: 10.1084/jem.138.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Polley M. J. Specificity of human lymphocyte complement receptors. J Exp Med. 1975 May 1;141(5):1163–1180. doi: 10.1084/jem.141.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Logue G. L., Adams J., Crookston J. H. Mechanisms of immune lysis of the red cells in hereditary erythroblastic multinuclearity with a positive acidified serum test and paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1974 Jan;53(1):31–43. doi: 10.1172/JCI107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F. Quantitative immunology of immune hemolytic anemia: II. The relationship of cell-bound antibody to hemolysis and the effect of treatment. J Clin Invest. 1971 Apr;50(4):734–743. doi: 10.1172/JCI106544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria E. J., Muchmore E. A., Sudora E. J., Masouredis S. P. The role of antigen mobility in anti-Rh0(D)-induced agglutination. J Clin Invest. 1975 Aug;56(2):292–301. doi: 10.1172/JCI108093. [DOI] [PMC free article] [PubMed] [Google Scholar]


