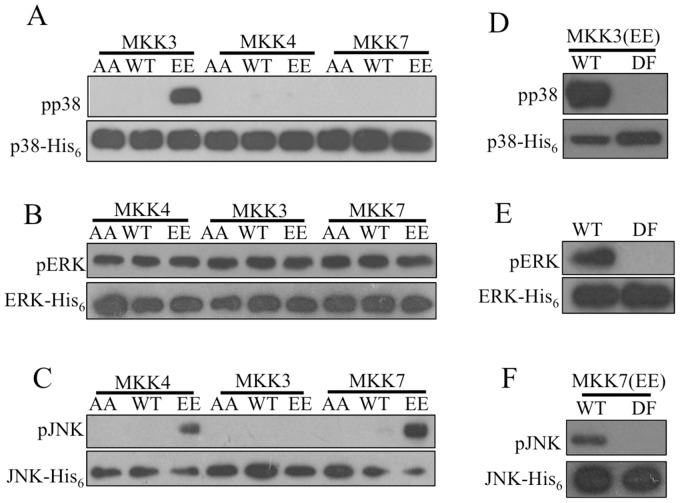
Figure 3. MKK3 specifically phosphorylated p38MAPK.
Constitutively activated or inactivated MKKs were constructed by replacing the dual phosphorylation sites with Glu and Ala, respectively. Phosphorylation-defective mutations of MAPKs were constructed by substituting the Thr-X-Tyr motif with Ala-X-Phe. MKKs and MAPKs were expressed in E. coli with a GST or His6 tag, respectively, and used for kinase assays. (A) Constitutively activated MKK3 effectively phosphorylated p38MAPK. (B) None of the MKKs enhanced activation of ERK, as similar intensity of signal was detected in all lanes. (C) Both constitutively activated MKK4 and MKK7 effectively phosphorylated JNK. (D) MKK3 phosphorylated wild type p38MAPK, but not the phosphorylation-defective mutant. (E) In the absence of MKK protein, wild type ERK was auto-phosphorylated, but no signal was detected when the phosphorylation-defective ERK was used. (F) Constitutively activated MKK7 significantly phosphorylated the wild type form of JNK but not the phosphorylation-defective form. AA: constitutively inactivated mutation; WT: wild type; EE: constitutively activated mutation; DF: phosphorylation-defective mutation.