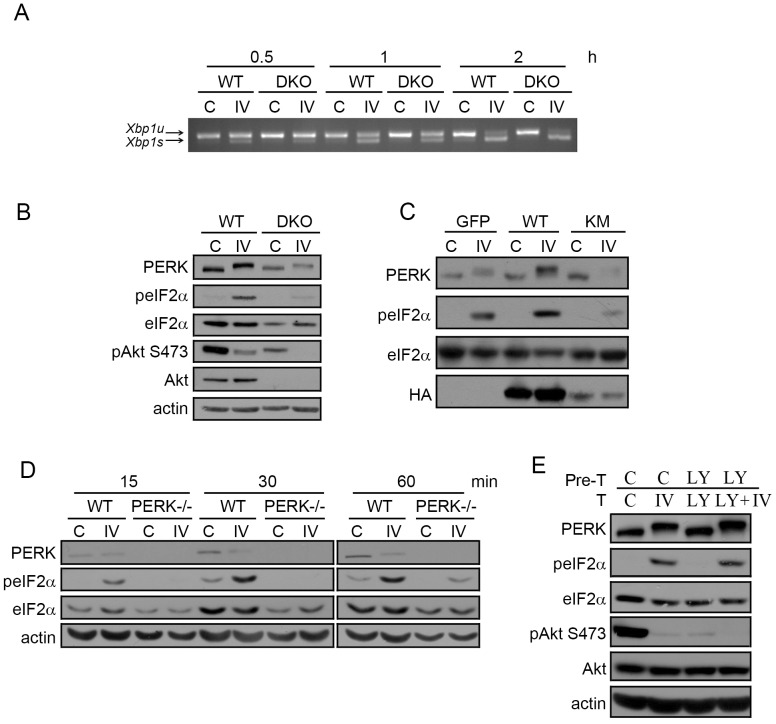
Figure 3. Akt-IV stimulation of eIF2α phosphorylation is Akt- and PERK- dependent but PI3K-independent.
(A) MEF WT or Akt DKO cells were treated with Akt-IV (IV; 10 µM) for the indicated times. Xbp1 mRNA splicing was detected by RT-PCR. Xbp1s: spliced form (activated IRE1); Xbp1u: unspliced form (inactive IRE1). (B) MEF WT or Akt DKO cells were treated with Akt-IV (IV; 10 µM) for 1 h. Protein extracts were analyzed by WB using the indicated antibodies. The fold change in peIF2α/eIF2α ratio induced by Akt-IV was quantified for three independent experiments. On average, this fold change was reduced to 15% of the original effect in MEF Akt DKO cells compared to WT cells (11.0 vs 2.6). (C) MEF WT cells were transfected with HA-Akt or HA-Akt KM plasmids. Forty-eight hours post-transfection cells were treated with DMSO (C) or with Akt-IV (IV; 10 µM) for 1 h. A GFP expressing plasmid was used as a transfection control. Protein extracts were analyzed by WB using the indicated antibodies. (D) MEFs WT or PERK−/− were treated with Akt-IV (IV; 10 µM) for the indicated times. Protein extracts were analyzed by WB using the indicated antibodies. (E) HEK293T cells were pretreated with DMSO (C) or LY294002 (LY; 20 µM) for 30 min and then treated for 1 h with DMSO, or Akt-IV (without removing the corresponding pre-treatment). Protein extracts were analyzed by WB using the indicated antibodies. Data are representative of at least three independent experiments.