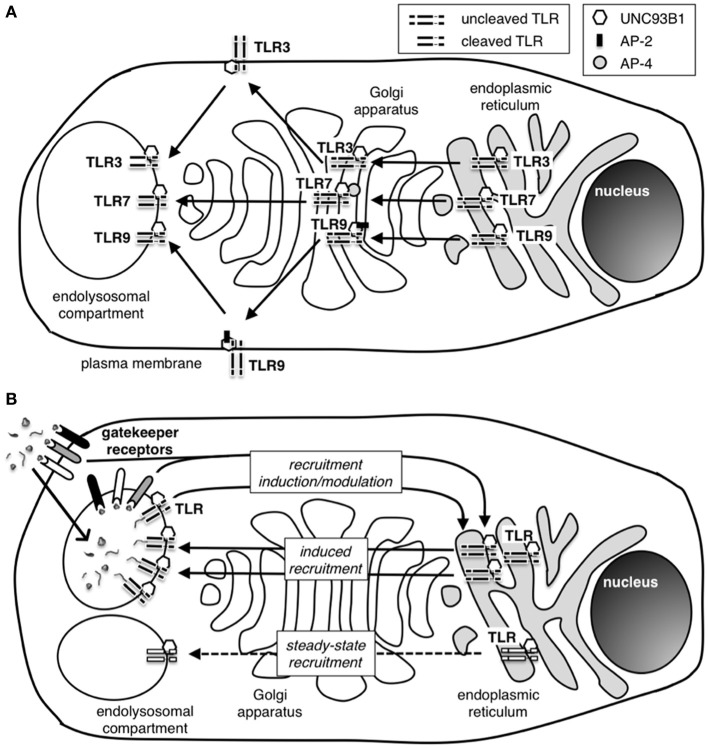
Figure 3.
How are nucleic acid-sensing TLR recruited to the endolysosomal compartment? (A) Nucleic acid sensing TLR traffic to the endolysosomal compartment from the endoplasmic reticulum (ER) via the Golgi apparatus. The chaperone protein UNC93B1 is necessary for successful migration of all three endosomal TLR, and adaptor protein (AP) complexes AP-2 an AP-4 are additionally required during the shuttle process of TLR9 and TLR7, respectively. TLR9, but not TLR7, is transiently located at the cell plasma membrane before reaching the endolysosome. Similarly, TLR3 has been detected at the cell surface in specific cell types. The reason for these differences in trafficking pathways for endosomal TLR is currently unknown. While in the ER and passing through the Golgi, TLR remain in an uncleaved state and only undergo cleavage to attain functional activation after arrival in the endolysosome. The requirement for cleavage is thought to be a protective mechanism to avoid unwanted TLR activation outside the endolysosomal compartment. (B) The induction and regulation of TLR recruitment to the endolysosomal compartment is still poorly understood. A small number of TLR have been detected in the endolysosomal compartment in the absence of activating stimuli suggesting the presence of a self-perpetuating low-frequency shuttling process of TLR to the endolysosome (steady-state recruitment). During infection, high frequency recruitment of TLR to the endolysosome takes place (induced recruitment). Induced recruitment is likely to be initiated by PAMP-activated TLR and/or other PRR. However, gatekeeper receptors controlling pathways such as the CD24-Siglec or CLR-Syk pathway have the ability to modulate and maybe even induce TLR recruitment to the endolysosomal compartment. The presence of gatekeeper receptors with the ability to distinguish specific signals from pathogens or uninfected host cells and to promote or regulate TLR recruitment is likely to play an important role in protecting the host from innate autoimmune activation.