Figure 4.
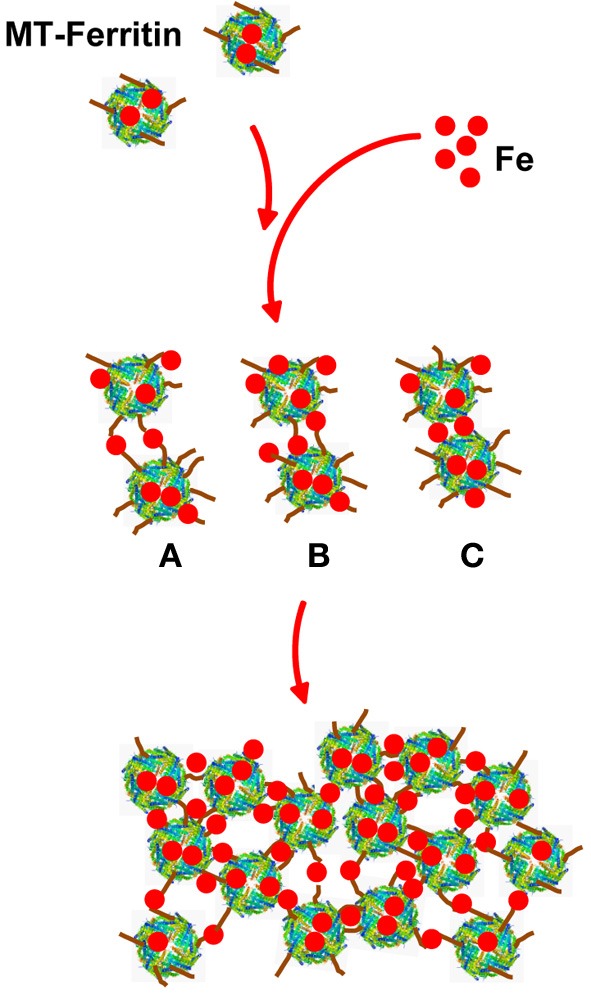
Model of iron-induced aggregation of ferritin containing the C-terminal mutation. Unraveled MT-FTL C-terminal E-helices (brown lines) can extend a substantial distance from the ferritin shell surface into the solvent providing a number of groups that can coordinate iron. Addition of iron to a solution of MT-FTL-containing ferritin initiates bridging of C-termini by iron (or iron nucleation complexes) reducing their translational motion. Cross-linking may occur between two separate ferritin 24-mers through iron bridges between C-termini (A), between a C-terminus and surface iron-binding amino acid side chain (B), and/or eventually through surface amino acids that bind iron on both 24-mers (C) forming ferritin aggregates [adapted from Baraibar et al. (2008)].
