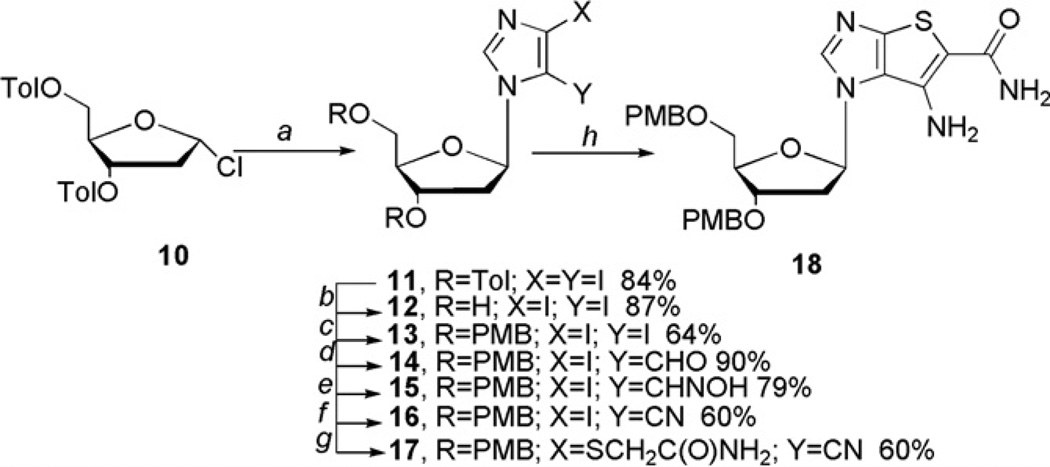
Scheme 2.
Synthesis of 2′-deoxy intermediate 18. Reagents and conditions: (a) MeCN, NaH, 30 min, diiodoimidazole, 24 h, rt ; (b) MeOH, NaOMe; (c) THF, NaH, 3 h, TBAI, PMBCl, 18 h, rt; (d) (i) EtMgBr, THF, 15 min; (ii) anhydrous DMF; (e) (i) NaHCO3, hydroxylamine hydrochloride, H2O; (ii) carbaldehyde, EtOH, rt, 18 h; (f) CDI, THF, reflux, 18 h; (g) NH2C(O)CH2SH, K2CO3, DMF, 60 °C; (h) NaOEt, EtOH.