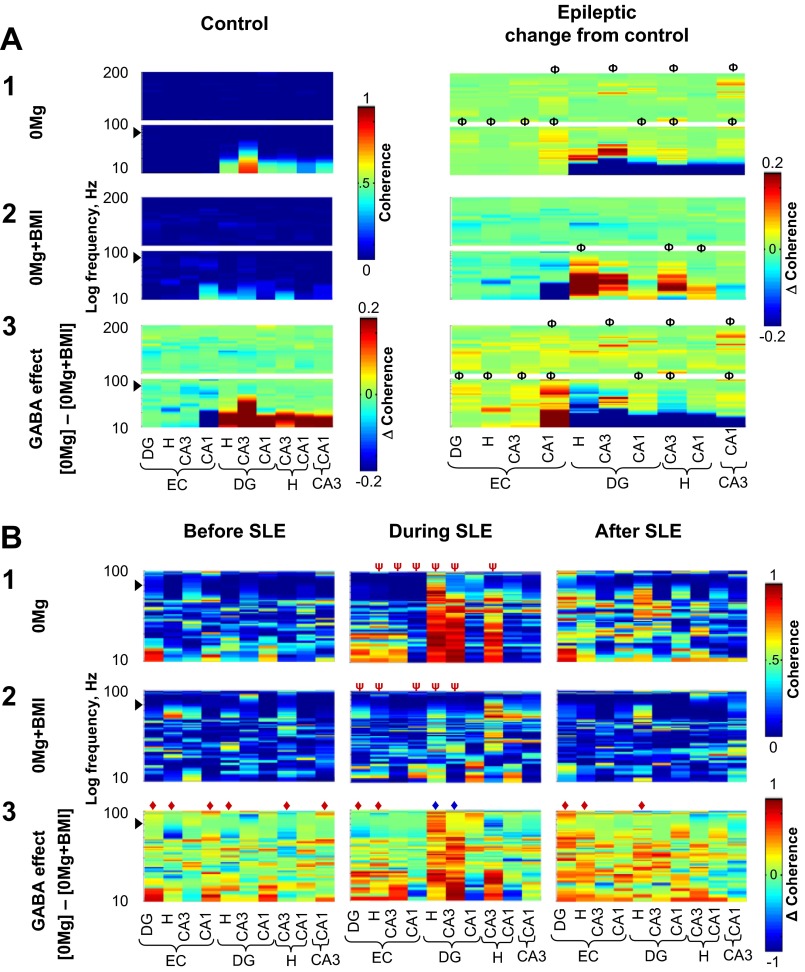
Fig. 6.
When excitatory transmission was enhanced, slow oscillation coherence was highest between the hilus, dentate gyrus, and CA3 and GABAergic transmission facilitated slow oscillation coherence between CA3, hilus, and dentate gyrus during SLE. Oscillation coherence in individual 0.5-s windows recorded from electrodes in different regions was calculated for slices from control (A, left) and epileptic (A, right, and B) rats; coherence measures from experimental groups were then averaged and plotted as described in materials and methods. Although stand-alone population spikes (∼7 ms in duration) were excluded from analysis, population spikes superimposed on suprathreshold slow oscillations may contaminate slow oscillation results at ∼71 Hz (arrowheads). The y-axis is stretched to yield equal height panels for each frequency range. Coherence measures for fast ripples (201 Hz–1 kHz) in A and B and ripples (101–200 Hz) in B are not shown because there were no dramatic changes in these oscillation ranges across experimental groups. A: coherence measures of each 0.5-s window recorded from different electrodes across different regions were compiled for all 0.5-s windows in the entire duration of recording. In both 0Mg (A1) and 0Mg+BMI (A2), slow oscillation coherence was highest between the dentate gyrus, hilus, and CA3 and this coherence was shifted to higher slow oscillation frequency bands in epileptic (0Mg, n = 11; 0Mg+BMI, n = 9) compared with control (0Mg, n = 7; 0Mg+BMI, n = 8) tissue. In 0Mg (A1), but not 0Mg+BMI (A2), bands of ripple coherence between CA3 and other hippocampal regions were increased in slices from epileptic compared with control rats. A3: subtraction of coherences in A1 and A2 revealed that in slices from control rats (left), when excitatory transmission was enhanced GABAergic transmission facilitated slow oscillation coherence between hippocampal regions and reduced slow oscillation coherence between the entorhinal cortex and CA1. Right: when excitatory transmission was enhanced, GABAergic transmission facilitated slow oscillation coherence between CA3 and the dentate gyrus and reduced slow oscillation coherence between the hilus, dentate gyrus, and CA3 to a greater degree in slices from epileptic compared with control rats. GABAergic transmission also facilitated ripple coherence between CA3 and other hippocampal subregions to a greater degree in epileptic rats. B: coherences were calculated for all 0.5-s windows in the 3 s immediately before SLE (left), the first 3 s of SLE (center), and the first 3 s after SLE termination (right) in slices from epileptic rats. The start of SLE was defined as described in materials and methods. In 0Mg (B1), but not 0Mg+BMI (B2), slow oscillation coherence between the dentate gyrus, hilus, and CA3 was increased during SLE compared with before and after SLE. B3: subtraction of coherence in B1 and B2 revealed that when excitatory transmission was enhanced GABAergic transmission facilitated oscillation coherence between the dentate gyrus, hilus and CA3 during SLE (center, blue ◆) compared with before and after SLE. ΦAt least 1 peak wider than 5 Hz different from control, ◆overall range difference between 0Mg and 0Mg+BMI, ψoverall range different than before and after SLE, P < 0.05, 2-way ANOVA with Sidak post hoc comparison. Scale bar in A1 and 2, left, applies to both left panels in A1 and 2; scale bar in A2, right, applies to all right panels in A1–3; scale bar in A3, left, applies only to that panel. Scale bar in B1 and 2, right, applies to all panels in B1 and 2; scale bar in B3, right, applies to all panels in B3.