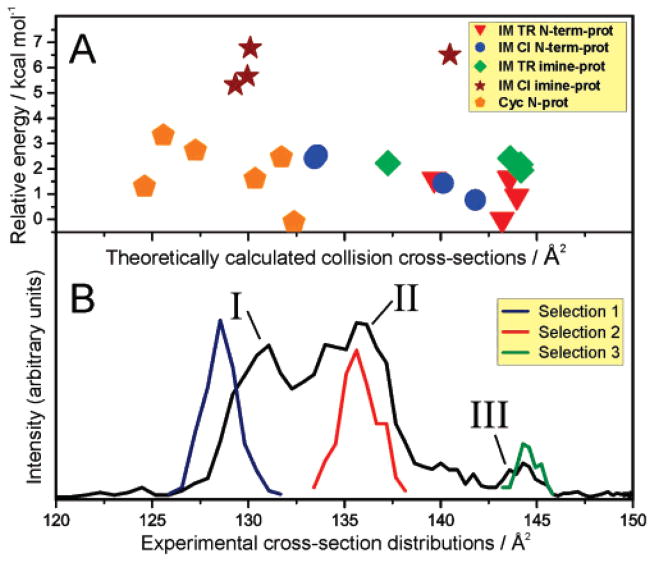
Figure 2.
(A) Theoretical cross-sections and relative energies for the density-functional theory calculated structures of the lowest-energy imine and cyclic variants of a4 for Leu-enkephalin. The legend indicates the different imine variants, showing the cis and trans isomers and the site of proton attachment. (B) Experimentally determined cross-section distribution of a4, based on the mobilities through the second drift tube. Mobility selections (1–3) were also carried out at the end of the second drift tube, as explained in the Methods. Note that the intensities of the mobility selections are increased by a factor of 40 relative to the unselected a4 distribution. The three distinct features in the mobility spectrum are labeled as I, II and III.