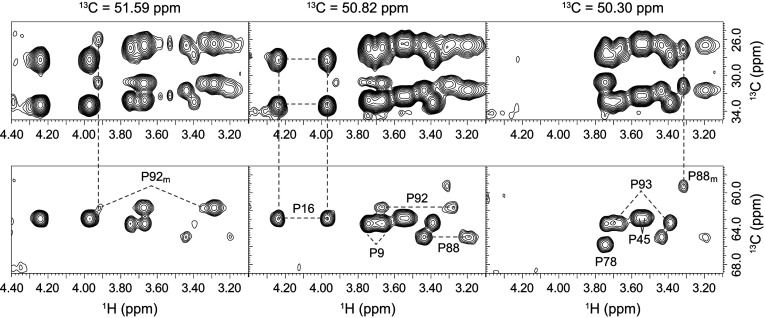
Figure 5. 3D (H)CCH-TOCSY correlation for the seven proline residues of FKBP12.
Spectral region for the proline 13Cβ and 13Cγ (upper panels) and 13Cα (lower panels) resonances that are correlated to the intraresidue 13Cδ resonances at three frequencies within a 3D (H)CCH-TOCSY experiment. Complete connectivity patterns are observed for all seven proline residues in the major slow exchange state and for the resolved resonances of Pro88 and Pro92 in the minor slow exchange state. The small (4.0 p.p.m.) chemical shift differences for 13Cβ–13Cγ indicate that these two proline residues remain in a trans-peptide conformation in the minor slow exchange state.