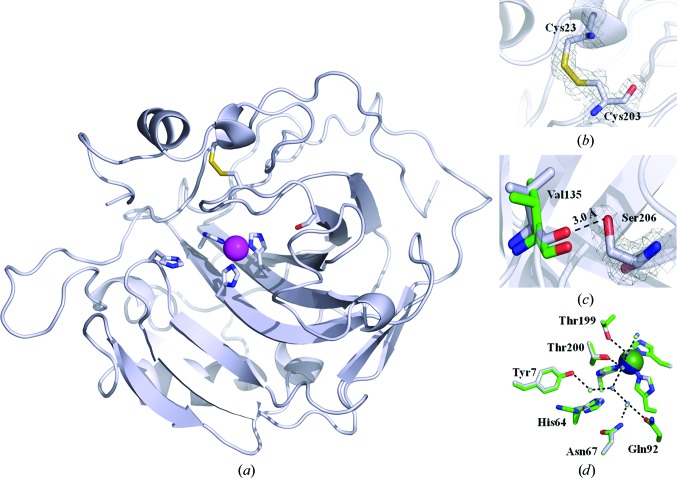
Figure 1.
X-ray crystallographic structure of dsHCAII. (a) Cartoon view of the overall topology of dsHCAII. The Cα backbone is shown in silver, with N atoms colored blue, O atoms red and S atoms yellow, and the Zn2+ ion represented as a magenta sphere. The coordinating histidine residues to the zinc metal, His64, Cys23, Cys203 and Ser206 are shown in stick view. (b) Enlarged view of the disulfide bridge formed between residues 23 and 203 with the 2F o − F c map contoured at 1.4σ shown in gray. (c) Enlarged view of the dual conformation of Ser206 with the 2F o − F c map contoured at 1.4σ shown in gray with the distance between the hydroxyl group of Ser206 and the backbone carbonyl of Val135 indicated. (d) Comparison of the active-site side chains involved in forming the water network (dashed lines) for dsHCAII (silver) and HCAII (green). The zinc metal is colored blue for dsHCAII and green for HCAII. The figures were generated and rendered with PyMOL (DeLano, 2002 ▶).