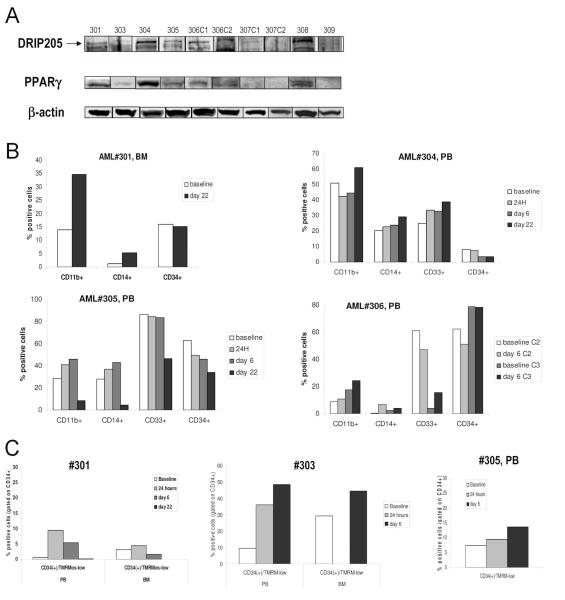
Fig. 6.
A. Peripheral blood (PB) or bone marrow (BM) samples from patients enrolled in the Phase 1 clinical trial were lysed and probed with DRIP205 and PPARγ by Western blot. β-actin was used as a loading control. In baseline sample from patient#2, not enough material was available for immunblotting. B. Patients were treated with CDDO (RTA-401) during a Phase I clinical trial, and cells were collected from the peripheral blood (PB) or bone marrow (BM) and assessed for expression of surface markers CD11b, CD14, CD33 and CD34 by flow cytometry at the indicated times (see also suppl. Table 2). Four of nine patients (patients #301, 304, 305, 306) showed alterations of these parameters during the observed period. PB baseline percentages are not available from patient #301, therefore, BM percentages are provided. C. Cells from the PB or BM were counterstained with CD34-APC and TMRM. Three of five patients (patients #301, 303, 305) showed alterations of these parameters during the observed period. Data are presented as percentage of CD34+ cells that have lost mitochondrial membrane potential (TMRM-low). BM baseline percentages are not available from patient #305.