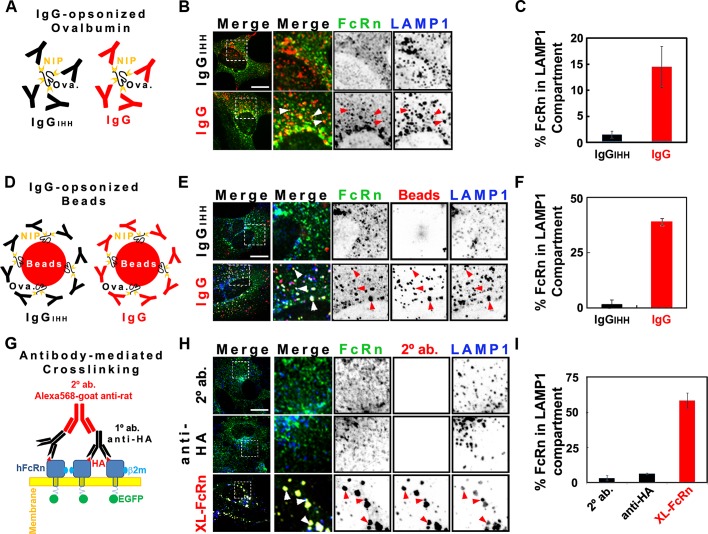
FIGURE 1:
Cross-linked HA-FcRn-EGFP is diverted from recycling endosomes to lysosomes. For indirect cross-linking experiments (see models A and D), HMEC-1 cells were incubated at pH 6 with 20 μM NIP-ovalbumin opsonized with IgG (1:5 M ratio; A–C) or 4.5 pM 100-nm IgG-opsonized fluorescent beads (1:100 beads-to-IgG molar ratio; D–F) for 3 h at 37°C. For direct cross-linking experiments (G–I), cells were incubated with HBSS-H buffer (row 1) or primary (rows 2 and 3) rat anti-HA antibodies (2 μg/ml) for 1 h at 37°C in HBSS-H (pH 7.4). Samples were washed with PBS buffer (pH 7.4) and further incubated with HBSS-H (row 2) or secondary (rows 1 and 3) Alexa 568–goat anti-rat antibodies (40 μg/ml) for 3 h at 37°C. Cells were washed, fixed, and stained for LAMP-1 (see Materials and Methods). Representative confocal middle sections of each condition are shown, with arrowheads indicating examples of colocalization between HA-FcRn-EGFP, ligands, and LAMP-1. Selected regions (stippled-line squares) were cropped and enlarged and are displayed using an inverted monochrome color scale to aid visualization. Scale bar, 10 μm. Bar plots (C, F, I) depict the quantification calculated by mask analysis (see Materials and Methods) of diversion of FcRn to the LAMP-1 compartment (average and SD of three to five independent experiments, n = 10–20 cells).