Abstract
Branched-chain fatty acids of the iso and anteiso series occur in many bacteria as the major acyl constituents of membrane lipids. In addition, omega-cyclohexyl and omega-cycloheptyl fatty acids are present in several bacterial species. These two types of fatty acids are synthesized by the repeated condensation of malonyl coenzyme A with one of the branched-chain and cyclic primers by the same enzyme system. The pathway of de novo branched-chain fatty acid synthesis differs only in initial steps of synthesis from that of the common straight-chain fatty acid (palmitic acid) present in most organisms. The cell membranes composed largely of iso-, anteiso-, and omega-alicyclic acids support growth of bacteria, which inhabit normal as well as extreme environments. The occurrence of these types of fatty acids as major cellular fatty acids is an important criterion used to aid identification and classification of bacteria.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Bell R. M., Vagelos P. R. Acyl carrier protein. XV. Studies of -ketoacyl-acyl carrier protein synthetase. J Biol Chem. 1972 May 25;247(10):3190–3198. [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballio A., Barcellona S. Relations chimiques et immunologiques chez les Actinomycétales. I. Les acides gras de 43 souches d'actinomycètes aérobies. Ann Inst Pasteur (Paris) 1968 Feb;114(2):121–137. [PubMed] [Google Scholar]
- Bloch K., Vance D. Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem. 1977;46:263–298. doi: 10.1146/annurev.bi.46.070177.001403. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Lehane D. P. The phospholipids of corynebacteria. Lipids. 1971 Jun;6(6):401–409. doi: 10.1007/BF02531377. [DOI] [PubMed] [Google Scholar]
- Butterworth P. H., Bloch K. Comparative aspects of fatty acid synthesis in Bacillus subtilis and Escherichia coli. Eur J Biochem. 1970 Feb;12(3):496–501. doi: 10.1111/j.1432-1033.1970.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Chan M., Himes R. H., Akagi J. M. Fatty acid composition of thermophilic, mesophilic, and psychrophilic clostridia. J Bacteriol. 1971 Jun;106(3):876–881. doi: 10.1128/jb.106.3.876-881.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clejan S., Krulwich T. A., Mondrus K. R., Seto-Young D. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J Bacteriol. 1986 Oct;168(1):334–340. doi: 10.1128/jb.168.1.334-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Goodfellow M., Minnikin D. E. Fatty acid composition of some mycolic acid-containing coryneform bacteria. J Gen Microbiol. 1982 Nov;128(11):2503–2509. doi: 10.1099/00221287-128-11-2503. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Goodfellow M., Minnikin D. E. Fatty acid, isoprenoid quinone and polar lipid composition in the classification of Curtobacterium and related taxa. J Gen Microbiol. 1980 May;118(1):29–37. doi: 10.1099/00221287-118-1-29. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Shah H. N., McKee A. S., Kroppenstedt R. M. Chemotaxonomy of the genus Capnocytophaga (Leadbetter, Holt & Socransky). J Appl Bacteriol. 1982 Jun;52(3):409–415. doi: 10.1111/j.1365-2672.1982.tb05071.x. [DOI] [PubMed] [Google Scholar]
- De Rosa M., Gambacorta A., Bu'lock J. D. Effects of pH and temperature on the fatty acid composition of bacillus acidocaldarius. J Bacteriol. 1974 Jan;117(1):212–214. doi: 10.1128/jb.117.1.212-214.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa M., Gambacorta A. The lipids of archaebacteria. Prog Lipid Res. 1988;27(3):153–175. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Dreher R., Poralla K., König W. A. Synthesis of omega-alicyclic fatty acids from cyclic precursors in Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1136–1140. doi: 10.1128/jb.127.3.1136-1140.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Zheng T., In't Veld G., Op den Kamp J. A., Konings W. N. Lipid requirement of the branched-chain amino acid transport system of Streptococcus cremoris. Biochemistry. 1988 Feb 9;27(3):865–872. doi: 10.1021/bi00403a005. [DOI] [PubMed] [Google Scholar]
- Drucker D. B. Chemotaxonomic fatty-acid fingerprints of some streptococci with subsequent statistical analysis. Can J Microbiol. 1974 Dec;20(12):1723–1728. doi: 10.1139/m74-266. [DOI] [PubMed] [Google Scholar]
- Ehret W., Jacob K., Ruckdeschel G. Identification of clinical and environmental isolates of Legionella pneumophila by analysis of outer-membrane proteins, ubiquinones and fatty acids. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Aug;266(1-2):261–275. doi: 10.1016/s0176-6724(87)80040-8. [DOI] [PubMed] [Google Scholar]
- Fautz E., Rosenfelder G., Grotjahn L. Iso-branched 2- and 3-hydroxy fatty acids as characteristic lipid constituents of some gliding bacteria. J Bacteriol. 1979 Dec;140(3):852–858. doi: 10.1128/jb.140.3.852-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche D., Thelen A. Die Abgrenzung der Genera Bacteroides und Sphaerophorus auf Grund der Struktur ihrer komplexen Lipoide. Zentralbl Bakteriol Orig A. 1973 Mar;223(2):356–365. [PubMed] [Google Scholar]
- Fulco A. J. Fatty acid metabolism in bacteria. Prog Lipid Res. 1983;22(2):133–160. doi: 10.1016/0163-7827(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Girard A. E. A comparative study of the fatty acids of some micrococci. Can J Microbiol. 1971 Dec;17(12):1503–1508. doi: 10.1139/m71-240. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Leadbetter E. R. Sulfonolipids of gliding bacteria. Structure of the N-acylaminosulfonates. J Biol Chem. 1984 Mar 10;259(5):2982–2990. [PubMed] [Google Scholar]
- Goodfellow M., Collins M. D., Minnikin D. E. Fatty acid and polar lipid composition in the classification of Kurthia. J Appl Bacteriol. 1980 Apr;48(2):269–276. doi: 10.1111/j.1365-2672.1980.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Doolittle W. F. Has the endosymbiont hypothesis been proven? Microbiol Rev. 1982 Mar;46(1):1–42. doi: 10.1128/mr.46.1.1-42.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan M. D., Alberts A. W., Vagelos P. R. Acyl carrier protein. 13. Beta-ketoacyl acyl carrier protein synthetase from Escherichia coli. J Biol Chem. 1969 Dec 10;244(23):6477–6485. [PubMed] [Google Scholar]
- HOFHEINZ W., GRISEBACH H. DIE FETTSAEUREN VON STREPTOMYCES ERYTHREUS UND STREPTOMYCES HALSTEDII. Z Naturforsch B. 1965 Jan;20:43–53. [PubMed] [Google Scholar]
- HORNING M. G., MARTIN D. B., KARMEN A., VAGELOS P. R. Fatty acid synthesis in adipose tissue. II. Enzymatic synthesis of branched chain and odd-numbered fatty acids. J Biol Chem. 1961 Mar;236:669–672. [PubMed] [Google Scholar]
- Herrero A. A., Gomez R. F., Roberts M. F. Ethanol-induced changes in the membrane lipid composition of Clostridium thermocellum. Biochim Biophys Acta. 1982 Dec 8;693(1):195–204. doi: 10.1016/0005-2736(82)90487-4. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Forcier G., Takacs B. J. Fatty acid composition of gliding bacteria: oral isolates of Capnocytophaga compared with Sporocytophaga. Infect Immun. 1979 Oct;26(1):298–304. doi: 10.1128/iai.26.1.298-304.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifkovits R. W., Ragheb H. S. Cellular fatty acid composition and identification of rumen bacteria. Appl Microbiol. 1968 Sep;16(9):1406–1413. doi: 10.1128/am.16.9.1406-1413.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Murphy C. M., Cronan J. E., Jr, Rock C. O. Acetoacetyl-acyl carrier protein synthase. A target for the antibiotic thiolactomycin. J Biol Chem. 1989 May 5;264(13):7624–7629. [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J Biol Chem. 1987 Jun 5;262(16):7927–7931. [PubMed] [Google Scholar]
- Jackson T. J., Ramaley R. F., Meinschein W. G. Fatty acids of a non-pigmented, thermophilic bacterium similar to Thermus aquaticus. Arch Mikrobiol. 1973;88(2):127–133. doi: 10.1007/BF00424766. [DOI] [PubMed] [Google Scholar]
- Jantzen E., Bergan T., Bovre K. Gas chromatography of bacterial whole cell methanolysates; VI. Fatty acid composition of strains within Micrococcaceae;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):785–798. [PubMed] [Google Scholar]
- Joseph R. Fatty acid composition of Spirochaeta stenostrepta. J Bacteriol. 1972 Oct;112(1):629–631. doi: 10.1128/jb.112.1.629-631.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
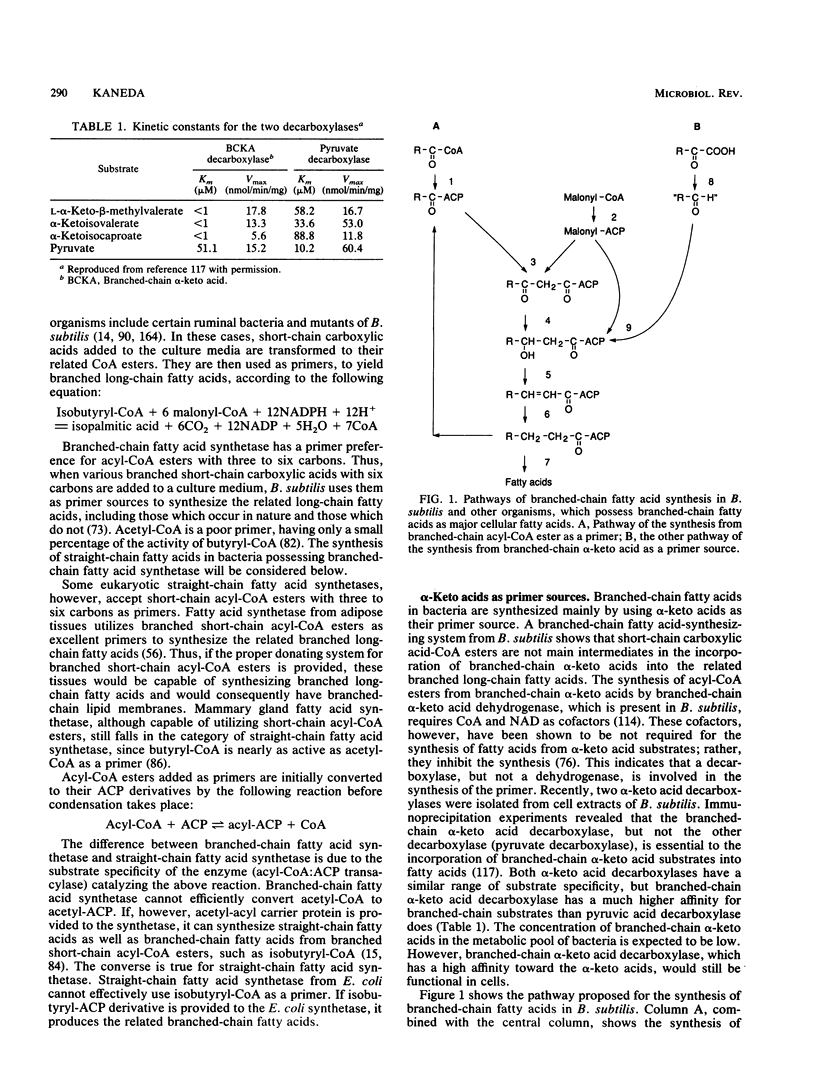
- Kaneda T. Biosynthesis of branched long-chain fatty acids from the related short-chain -keto acid substrates by a cell-free system of Bacillus subtilis. Can J Microbiol. 1973 Jan;19(1):87–96. doi: 10.1139/m73-013. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. V. Microbial stereospecific syntheses of D-12-methyltetradecanoic and D-14-methylhexadecanoic acids. Biochim Biophys Acta. 1966 Aug 3;125(1):43–54. doi: 10.1016/0005-2760(66)90142-1. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J Bacteriol. 1967 Mar;93(3):894–903. doi: 10.1128/jb.93.3.894-903.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. II. Similarity in the fatty acid compositions of Bacillus thuringiensis, Bacillus anthracis, and Bacillus cereus. J Bacteriol. 1968 Jun;95(6):2210–2216. doi: 10.1128/jb.95.6.2210-2216.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev. 1977 Jun;41(2):391–418. doi: 10.1128/br.41.2.391-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Incorporation of branched-chain C6-fatty acid isomers into the related long-chain fatty acids by growing cells of Bacillus subtilis. Biochemistry. 1971 Jan 19;10(2):340–347. doi: 10.1021/bi00778a022. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Major occurrence of cis-delta 5 fatty acids in three psychrophilic species of Bacillus. Biochem Biophys Res Commun. 1971 Apr 16;43(2):298–302. doi: 10.1016/0006-291x(71)90752-2. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Positional distribution of fatty acids in phospholipids from Bacillus subtilis. Biochim Biophys Acta. 1972 May 23;270(1):32–39. doi: 10.1016/0005-2760(72)90174-9. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Positional preference of fatty acids in phospholipids of Bacillus cereus and its relation to growth temperature. Biochim Biophys Acta. 1972 Oct 5;280(2):297–305. doi: 10.1016/0005-2760(72)90097-5. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Seasonal population changes and characterization of ice-nucleating bacteria in farm fields of central alberta. Appl Environ Microbiol. 1986 Jul;52(1):173–178. doi: 10.1128/aem.52.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T., Smith E. J., Naik D. N. Fatty acid composition and primer specificity of de novo fatty acid synthetase in Bacillus globispores, Bacillus insolitus, and Bacillus psychrophilus. Can J Microbiol. 1983 Dec;29(12):1634–1641. doi: 10.1139/m83-250. [DOI] [PubMed] [Google Scholar]
- Kaneda T., Smith E. J. Relationship of primer specificity of fatty acid de novo synthetase to fatty acid composition in 10 species of bacteria and yeasts. Can J Microbiol. 1980 Aug;26(8):893–898. doi: 10.1139/m80-155. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Stereoselective synthesis of chaulmoogric acid and related fatty acid from 2-(+/-)-cyclopentenecarboxylic acid by bacillus subtilis (ATCC 7059). Biochem Biophys Res Commun. 1981 Apr 30;99(4):1226–1229. doi: 10.1016/0006-291x(81)90750-6. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Stereoselectivity in the 2-methylbutyrate incorporation into anteiso fatty acids in Bacillus subtilis mutants. Biochim Biophys Acta. 1988 May 2;960(1):10–18. doi: 10.1016/0005-2760(88)90003-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Uemura N., Okuda S. Characterization of the fatty acid synthetase system of Curtobacterium pusillum. J Biochem. 1986 Jun;99(6):1735–1742. doi: 10.1093/oxfordjournals.jbchem.a135650. [DOI] [PubMed] [Google Scholar]
- Kawanami J. Lipids of Streptomyces toyocaensis. On the structure of siolipin. Chem Phys Lipids. 1971 Nov;7(3):159–172. doi: 10.1016/0009-3084(71)90029-6. [DOI] [PubMed] [Google Scholar]
- Knudsen J., Grunnet I. Primer specificity of mammalian mammary gland fatty acid synthetases. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1808–1814. doi: 10.1016/s0006-291x(80)80109-4. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Clejan S., Falk L. H., Guffanti A. A. Incorporation of specific exogenous fatty acids into membrane lipids modulates protonophore resistance in Bacillus subtilis. J Bacteriol. 1987 Oct;169(10):4479–4485. doi: 10.1128/jb.169.10.4479-4485.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsman J. E. Characterization of the lipids of six strains of Bacteroides ruminicola. J Bacteriol. 1973 Mar;113(3):1121–1126. doi: 10.1128/jb.113.3.1121-1126.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. S., Sarvas M., Brammar W. J., Neugebauer K., Wu H. C. Bacillus licheniformis penicillinase synthesized in Escherichia coli contains covalently linked fatty acid and glyceride. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3506–3510. doi: 10.1073/pnas.78.6.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Ferguson K. P., Mayberry W. R. Characterization of Bacteroides gingivalis by direct fluorescent antibody staining and cellular fatty acid profiles. Can J Microbiol. 1982 Apr;28(4):367–374. doi: 10.1139/m82-056. [DOI] [PubMed] [Google Scholar]
- Lambert M. A., Armfield A. Y. Differentiation of Peptococcus and Peptostreptococcus by gas-liquid chromatography of cellular fatty acids and metabolic products. J Clin Microbiol. 1979 Oct;10(4):464–476. doi: 10.1128/jcm.10.4.464-476.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechevalier M. P. Lipids in bacterial taxonomy - a taxonomist's view. CRC Crit Rev Microbiol. 1977;5(2):109–210. doi: 10.3109/10408417709102311. [DOI] [PubMed] [Google Scholar]
- Legendre S., Letellier L., Shechter E. Influence of lipids with branched-chain fatty acids on the physical, morphological and functional properties of Escherichia coli cytoplasmic membrane. Biochim Biophys Acta. 1980 Nov 18;602(3):491–505. doi: 10.1016/0005-2736(80)90328-4. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing omega-cyclohexyl fatty acids. Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1985 Aug 27;24(18):4903–4911. doi: 10.1021/bi00339a027. [DOI] [PubMed] [Google Scholar]
- Livermore B. P., Johnson R. C. Lipids of the Spirochaetales: comparison of the lipids of several members of the genera Spirochaeta, Treponema, and Leptospira. J Bacteriol. 1974 Dec;120(3):1268–1273. doi: 10.1128/jb.120.3.1268-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Isolation and characterization of an ornithine-containing lipid from Desulfovibrio gigas. J Bacteriol. 1975 Aug;123(2):523–529. doi: 10.1128/jb.123.2.523-529.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmet D., Bornstein N., Fleurette J. Identification des Legionella par analyse des acides gras en chromatographie phase gazeuse (CPG) et des ubiquinones en chromatographie liquide haute performance (CLHP). Ann Biol Clin (Paris) 1988;46(6):371–375. [PubMed] [Google Scholar]
- Mayberry W. R. Hydroxy fatty acids in Bacteroides species: D-(--)-3-hydroxy-15-methylhexadecanoate and its homologs. J Bacteriol. 1980 Aug;143(2):582–587. doi: 10.1128/jb.143.2.582-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N., Souza K. A. The relationship between environmental temperature, cell growth and the fluidity and physical state of the membrane lipids in Bacillus stearothermophilus. Biochim Biophys Acta. 1976 Sep 7;443(3):348–359. doi: 10.1016/0005-2736(76)90455-7. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J Mol Biol. 1974 Mar 25;84(1):145–157. doi: 10.1016/0022-2836(74)90218-6. [DOI] [PubMed] [Google Scholar]
- Meyer H., Meyer F. Lipid metabolism in the parasitic and free-living spirochetes Treponema pallidum (Reiter) and Treponema zuelzerae. Biochim Biophys Acta. 1971 Feb 2;231(1):93–106. doi: 10.1016/0005-2760(71)90257-8. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Dowell V. R., Jr, Farshtchi D., Raines L. J., Cherry W. B. Cultural characteristics and fatty acid composition of propionibacteria. J Bacteriol. 1969 Feb;97(2):561–570. doi: 10.1128/jb.97.2.561-570.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik D. N., Kaneda T. Biosynthesis of branched long-chain fatty acids by species of Bacillus: relative activity of three alpha-keto acid substrates and factors affecting chain length. Can J Microbiol. 1974 Dec;20(12):1701–1708. doi: 10.1139/m74-263. [DOI] [PubMed] [Google Scholar]
- Namba Y., Yoshizawa K., Ejima A., Hayashi T., Kaneda T. Coenzyme A- and nicotinamide adenine dinucleotide-dependent branched chain alpha-keto acid dehydrogenase. I. Purification and properties of the enzyme from Bacillus subtilis. J Biol Chem. 1969 Aug 25;244(16):4437–4447. [PubMed] [Google Scholar]
- O'Donnell A. G., Nahaie M. R., Goodfellow M., Minnikin D. E., Hájek V. Numerical analysis of fatty acid profiles in the identification of staphylococci. J Gen Microbiol. 1985 Aug;131(8):2023–2033. doi: 10.1099/00221287-131-8-2023. [DOI] [PubMed] [Google Scholar]
- Oku H., Kaneda T. Biosynthesis of branched-chain fatty acids in Bacillus subtilis. A decarboxylase is essential for branched-chain fatty acid synthetase. J Biol Chem. 1988 Dec 5;263(34):18386–18396. [PubMed] [Google Scholar]
- Oshima M., Ariga T. Omega-cyclohexyl fatty acids in acidophilic thermophilic bacteria. Studies on their presence, structure, and biosynthesis using precursors labeled with stable isotopes and radioisotopes. J Biol Chem. 1975 Sep 10;250(17):6963–6968. [PubMed] [Google Scholar]
- Oshima M., Miyagawa A. Comparative studies on the fatty acid composition of moderately and extremely thermophilic bacteria. Lipids. 1974 Jul;9(7):476–480. doi: 10.1007/BF02534274. [DOI] [PubMed] [Google Scholar]
- Pandhi P. N., Hammond B. F. A glycolipid from Rothia dentocariosa. Arch Oral Biol. 1975 May-Jun;20(5-6):399–401. doi: 10.1016/0003-9969(75)90035-7. [DOI] [PubMed] [Google Scholar]
- Raines L. J., Moss C. W., Farshtchi D., Pittman B. Fatty acids of Listeria monocytogenes. J Bacteriol. 1968 Dec;96(6):2175–2177. doi: 10.1128/jb.96.6.2175-2177.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilfors L., Wieslander A., Ståhl S. Lipid and protein composition of membranes of Bacillus megaterium variants in the temperature range 5 to 70 degrees C. J Bacteriol. 1978 Sep;135(3):1043–1052. doi: 10.1128/jb.135.3.1043-1052.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Silvius J. R., McElhaney N. Membrane lipid biosynthesis in Acholeplasma laidlawii B: de novo biosynthesis of saturated fatty acids by growing cells. J Bacteriol. 1977 Nov;132(2):497–504. doi: 10.1128/jb.132.2.497-504.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Shaw N., Stead D. A study of the lipid composition of Microbacterium thermosphactum as a guide to its taxonomy. J Appl Bacteriol. 1970 Sep;33(3):470–473. doi: 10.1111/j.1365-2672.1970.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ladenson R. C., Honegger J. L. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973 Jul 6;311(3):349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- Steinick L. E., Christiansson A. Adsorption of mycoplasmavirus MV-L2 to Acholeplasma laidlawii: effects of changes in the acyl-chain composition of membrane lipids. J Virol. 1986 Nov;60(2):525–530. doi: 10.1128/jvi.60.2.525-530.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayon R. A., Carroll K. K. Effect of growth conditions on the fatty acid composition of Listeria monocytogenes and comparison with the fatty acids of Erysipelothrix and Corynebacterium. Lipids. 1971 Nov;6(11):820–825. doi: 10.1007/BF02531211. [DOI] [PubMed] [Google Scholar]
- Tomimura E., Zeman N. W., Frankiewicz J. R., Teague W. M. Description of Bacillus naganoensis sp. nov. Int J Syst Bacteriol. 1990 Apr;40(2):123–125. doi: 10.1099/00207713-40-2-123. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Tunlid A., Hoitink H. A., Low C., White D. C. Characterization of bacteria that suppress rhizoctonia damping-off in bark compost media by analysis of Fatty Acid biomarkers. Appl Environ Microbiol. 1989 Jun;55(6):1368–1374. doi: 10.1128/aem.55.6.1368-1374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunlid A., Schultz N. A., Benson D. R., Steele D. B., White D. C. Differences in fatty acid composition between vegetative cells and N(2)-fixing vesicles of Frankia sp. strain CpI1. Proc Natl Acad Sci U S A. 1989 May;86(9):3399–3403. doi: 10.1073/pnas.86.9.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta N., Yamakawa T. Gaschromatographic studies of microbial components. 3. Research on precursor of branched chain fatty acids of Staphylococcus aureus and Streptomyces lavenduulae. Jpn J Exp Med. 1968 Oct;38(5):347–355. [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Uratani Y., Wakayama N., Hoshino T. Effect of lipid acyl chain length on activity of sodium-dependent leucine transport system in Pseudomonas aeruginosa. J Biol Chem. 1987 Dec 15;262(35):16914–16919. [PubMed] [Google Scholar]
- Verhulst A., Van Hespen H., Symons F., Eyssen H. Systematic analysis of the long-chain components of Eubacterium lentum. J Gen Microbiol. 1987 Feb;133(2):275–282. doi: 10.1099/00221287-133-2-275. [DOI] [PubMed] [Google Scholar]
- Verkest V., McArthur M., Hamilton S. Fatty acid activation of protein kinase C: dependence on diacylglycerol. Biochem Biophys Res Commun. 1988 Apr 29;152(2):825–829. doi: 10.1016/s0006-291x(88)80112-8. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Ware J. C., Dworkin M. Fatty acids of Myxococcus xanthus. J Bacteriol. 1973 Jul;115(1):253–261. doi: 10.1128/jb.115.1.253-261.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A., Heinen W. Effect of temperature on the fatty acid composition of the extreme thermophiles, Bacillus caldolyticus and Bacillus caldotenax. J Bacteriol. 1972 Jan;109(1):443–446. doi: 10.1128/jb.109.1.443-446.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L., De Siervo A. J., Salton M. R. Use of antibody to membrane adenosine triphosphatase in the study of bacterial relatioships. J Bacteriol. 1971 Mar;105(3):957–967. doi: 10.1128/jb.105.3.957-967.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Fatty acid-requiring mutant of bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971 Sep 10;246(17):5264–5272. [PubMed] [Google Scholar]
- Wollenweber H. W., Rietschel E. T., Hofstad T., Weintraub A., Lindberg A. A. Nature, type of linkage, quantity, and absolute configuration of (3-hydroxy) fatty acids in lipopolysaccharides from Bacteroides fragilis NCTC 9343 and related strains. J Bacteriol. 1980 Dec;144(3):898–903. doi: 10.1128/jb.144.3.898-903.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Z., Raynor R. L., Wood M. G., Jr, Menger F. M., Kuo J. F. Structure-activity relationship of synthetic branched-chain distearoylglycerol (distearin) as protein kinase C activators. Biochemistry. 1988 Sep 20;27(19):7361–7365. doi: 10.1021/bi00419a028. [DOI] [PubMed] [Google Scholar]



