Abstract
Persistent organic pollutants such as polychlorinated biphenyls (PCBs) are associated with detrimental health outcomes including cardiovascular diseases. Remediation of these compounds is a critical component of environmental policy. Although remediation efforts aim to completely remove toxicants, little is known about the effects of potential remediation byproducts. We previously published that Fe/Pd nanoparticles effectively dechlorinate PCB 77 to biphenyl, thus eliminating PCB-induced endothelial dysfunction using primary vascular endothelial cells. Herein, we analyzed the toxic effects of PCB congener mixtures (representative mixtures of commercial PCBs based on previous dechlorination data) produced at multiple time points during the dechlorination of PCB 77 to biphenyl. Compared with pure PCB 77, exposing endothelial cells to lower chlorinated PCB byproducts led to improved cellular viability, decreased superoxide production, and decreased nuclear factor kappa B activation based on duration of remediation. Presence of the parent compound, PCB 77, led to significant increases in mRNA and protein inflammatory marker expression. These data implicate that PCB dechlorination reduces biological toxicity to vascular endothelial cells.
Keywords: Dechlorination, Endothelial cell dysfunction, Oxidative stress, PCBs, Pollutant remediation
Introduction
Polychlorinated biphenyls (PCBs) are a class of organic pollutants that were used heavily as dielectric fluids in industrial and electrical applications until evidence surfaced linking PCBs to cancer and cardiovascular disease. Remediation efforts for these highly persistent pollutants have proven difficult, expensive, and slow. Our laboratories have developed new fast, low-cost techniques that employ Pd/Fe nanoparticles for reductive PCB dechlorination, which systematically dechlorinate higher congener PCBs to biphenyl, with lower chlorinated congeners produced as byproducts prior to complete degradation. The goal of this work is to better understand the toxicological effects of these byproducts and to further validate the importance of PCB dechlorination for reducing cardiovascular risk factors and for improving overall public health.
Even though PCB production has been banned in the US since the late 1970s, PCBs remain concentrated in many waterways and Superfund sites, as well as in electrical devices such as transformers and light ballasts. Additionally, recent research has indicated that a variety of PCB congeners are still being generated as industrial byproducts from pigment production and certain paper manufacturing (Rodenburg et al. 2010; Hu and Hornbuckle 2010). PCBs exhibit unique properties, such as high environmental persistence and resistance to metabolism in organisms, which lead to difficulties for remediation. Additionally, the costs associated with large-scale cleanup and removal of these and other chemical pollutants is great, and thus the development of more efficient, cost-effective technologies continues to be important. Current remediation techniques rely on expensive dredging followed by incineration or bioremediation techniques. Although PCBs can be degraded through some natural processes such as bacterial degradation (Brown et al. 1987; Rodenburg et al. 2012) and photolysis (Ruzo et al. 1974; Hawari et al. 1992; Doskey and Andren 1981), these processes are prohibitively slow for remediation of larger scale contaminations. Our research group recently developed a Pd/Fe nanoparticle system that catalyzes the dechlorination of PCBs to form biphenyl in order to address this need for low-cost, high-throughput chlorinated aromatic degradation (Zahran et al. 2011; Venkatachalam et al. 2008). During chlorinated aromatic degradation, hydrogen ions are used to displace chlorine on biphenyl rings in stages, resulting in the production of lower chlorinated PCB congeners prior to complete degradation to biphenyl. It is generally believed that less-chlorinated congeners are more water soluble, more volatile, and more likely to biodegrade further (Beyer and Biziuk 2009). In a previous study, we compared the biological activity of the parent compound (PCB 77) with the dechlorination end product (biphenyl) and found that complete dechlorination markedly reduced the pro-inflammatory activity of PCB 77 (Venkatachalam et al. 2008).
Many environmental contaminants, and especially persistent organic pollutants, are risk factors for cardiovascular diseases such as atherosclerosis because they can initiate or exacerbate the underlying disease by altering gene expression patterns and subsequent vascular inflammation (Puga et al. 2004; Hennig et al. 2007). Because the endothelium is in immediate contact with the blood, endothelial cells are particularly susceptible to the effect of environmental contaminants. The lining of blood vessels is protected by the endothelium, and endothelial cells play an active role in physiological processes such as regulation of vessel tone, blood coagulation, and vascular permeability. In turn, endothelial cell activation or dysfunction is a critical marker of the pathology of cardiovascular diseases such as atherosclerosis.
Activated endothelial cells produce chemokines such as monocyte chemoattractant protein 1 (MCP-1) and inflammatory cytokines, which attract monocytes from the blood stream to the site of injury. Part of the resulting inflammatory process includes the endothelial cell expression of adhesion molecules, like vascular adhesion molecule-1 (VCAM-1), which allow the monocytes to attach to the endothelial layer and infiltrate this barrier into the intimal space. We have demonstrated previously that coplanar PCBs (e.g., PCB 77) can induce DNA-binding activity of the oxidative stress-sensitive transcription factor nuclear factor kappa B (NFκB) and expression of VCAM-1, which is dependent on functional aryl hydrocarbon receptor (AhR) activity (Hennig et al. 2002). Recently, we have reported the mechanisms of PCB 77-mediated upregulation of MCP-1 (Majkova et al. 2009).
Little is known about vascular toxicity of PCBs and especially about the toxicity of dechlorination or remediation products. There is a clear need to evaluate products of remediation (e.g., dechlorination products) for biological activity in mammalian systems (Ganey and Boyd 2005). It is important to know to what degree remediation processes of persistent organic pollutants have to occur before toxicity, and any vascular injury becomes negligible. Thus, the present study was designed to test the effects of PCB 77 dechlorination products on pro-inflammatory parameters in a vascular endothelial cell model system. Data from this study suggest that the presence of the parent compound (e.g., PCB 77) is necessary for maximal endothelial cell dysfunction and inflammation. These data also suggest that dechlorination is a successful method for decreasing biological toxicity, with overall toxicity linked to the level of degradation of the parent compound. Herein, dechlorination is demonstrated to be an effective platform for addressing public health concerns associated with these persistent chlorinated pollutants.
Materials and methods
Biphenyl, 3-chlorobiphenyl (PCB 2), 4-chlorobiphenyl (PCB 3), 3,3′-dichlorobiphenyl (PCB 11), 3,4-dichlorobiphenyl (PCB 12), 3,4′-dichlorobiphenyl (PCB 13), 4,4′-dichloro biphenyl (PCB 15), 3, 3′,4-trichlorobiphenyl (PCB 35), 3,4,4′-trichlorobiphenyl (PCB 37), and 3, 3′, 4, 4′-tetrachloro biphenyl (PCB 77) were purchased from AccuStandard, Inc. (New Haven, CT). Experiments that included hazardous materials such as PCBs were performed in accordance with institutional and federal guidelines (Environmental Protection 2012). All cell culture reagents, including ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI), were purchased from Life Technologies (Carlsbad, CA). All other chemicals were purchased from Sigma-Aldrich Corporation (St. Louis, MO), unless otherwise specified.
Description of PCB 77 dechlorination treatment mixtures
Treatment mixtures were based on the composition of PCB 77 and its dechlorination products at 0, 10, 24, and 48 h of remediation as derived from gas chromatography-mass spectrometry data comparing the reductive efficiencies of 0.1 mg mL−1 400 nm Pd/Fe nanotubes and 1 mgmL−1 Pd/Fe nanoparticles (Zahran et al. 2011; Venkatachalam et al. 2008). Dechlorination product compositions were determined from these data (Table 1); treatments from each stage of the dechlorination are described as percent by weight and as molarity. Stocks of individual PCB commercial congeners were solubilized in fresh dimethyl sulfoxide (DMSO) at a concentration of 10 mM. Treatment mixtures were developed to delineate between the effects of the parent compound and dechlorination products including treatments representing product mixtures without PCB 77, treatments representing only the concentration of PCB 77 at a particular stage of dechlorination, and a treatment representing the 24 h dechlorination products with the higher PCB 77 concentration found following 10 h dechlorination, to emphasize the role of PCB 77 in overall toxicity (Table 2). Stock treatment mixtures were prepared in DMSO at a 5 mM total concentration and diluted 1:1,000 in cell culture medium for 5 μM final cell culture treatments.
Table 1.
Treatment mixtures representing PCB 77 dechlorination byproducts at various time points during dechlorination
| Mixture | DMSO | 0 h | 10 h | 24 h | 48 h |
|---|---|---|---|---|---|
| Components | (Vehicle) | (5 μM PCB 77) wt% (μM) | (5 μM dechlor. product) wt% (μM) | (5 μM dechlor. product) wt% (μM) | (5 μM biphenyl) wt% (μM) |
| PCB 77 | 100 % (5) | 20 % (0.7) | 7 % (0.2) | 0 % | |
| PCB 37 | 0 % | 4 % (0.2) | 2 % (0.1) | 0 % | |
| PCB 35 | 0 % | 4 % (0.2) | 2 % (0.1) | 0 % | |
| PCB 15 | 0 % | 11 % (0.5) | 9 % (0.4) | 0 % | |
| PCB 13 | 0 % | 8 % (0.3) | 7 % (0.3) | 0 % | |
| PCB 12 | 0 % | 8 % (0.3) | 7 % (0.3) | 0 % | |
| PCB 11 | 0 % | 11 % (0.5) | 9 % (0.4) | 0 % | |
| PCB 03 | 0 % | 3 % (0.1) | 3 % (0.1) | 0 % | |
| PCB 02 | 0 % | 3 % (0.1) | 3 % (0.1) | 0 % | |
| Biphenyl | 0 % | 30 % (1.9) | 50 % (3.1) | 100 % (5) | |
| Total | 100 % (5) | 100 % (5) | 100 % (5) | 100 % (5) |
Table 2.
Original and modified treatment conditions based on PCB 77 dechlorination byproducts
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mixture components | DMSOa (0.1 %v/v vehicle) | 0 ha [μM (ppm)] | 10 ha [μM (ppm)] | 10 h without PCB 77b [μM (ppm)] | 24 ha [μM (ppm)] | 24 h without PCB 77b [μM (ppm)] | 24 h with addition of 10 h PCB 77c [μM (ppm)] | 48 ha [μM (ppm)] | 10 h Concentration of PCB 77 d [μM (ppm)] | 24 h Concentration of PCB 77d [μM (ppm)] |
| PCB 77 | – | 5 (1.46) | 0.7 (0.200) | – | 0.2 (0.064) | – | 0.7 (0.200) | – | 0.7 (0.200) | 0.2 (0.064) |
| PCB 37 | – | – | 0.2 (0.042) | 0.2 (0.042) | 0.1 (0.020) | 0.1 (0.020) | 0.1 (0.020) | – | – | – |
| PCB 35 | – | – | 0.2 (0.042) | 0.2 (0.042) | 0.1 (0.020) | 0.1 (0.020) | 0.1 (0.020) | – | – | – |
| PCB 15 | – | – | 0.5 (0.110) | 0.5 (0.110) | 0.4 (0.090) | 0.4 (0.090) | 0.4 (0.090) | – | – | – |
| PCB 13 | – | – | 0.3 (0.076) | 0.3 (0.076) | 0.3 (0.070) | 0.3 (0.070) | 0.3 (0.070) | – | – | – |
| PCB 12 | – | – | 0.3 (0.076) | 0.3 (0.076) | 0.3 (0.070) | 0.3 (0.070) | 0.3 (0.070) | – | – | – |
| PCB 11 | – | – | 0.5 (0.110) | 0.5 (0.110) | 0.4 (0.090) | 0.4 (0.090) | 0.4 (0.090) | – | – | – |
| PCB 03 | – | – | 0.1 (0.028) | 0.1 (0.028) | 0.1 (0.024) | 0.1 (0.024) | 0.1 (0.024) | – | – | – |
| PCB 02 | – | – | 0.1 (0.028) | 0.1 (0.028) | 0.1 (0.024) | 0.1 (0.024) | 0.1 (0.024) | – | – | – |
| Biphenyl | – | – | 1.9 (0.300) | 1.9 (0.300) | 3.1 (0.480) | 3.1 (0.480) | 3.1 (0.480) | 5 (0.771) | – | – |
| Total Concentration | – | 5 (1.46) | 5 (1.01) | 4.3 (0.810) | 5 (0.954) | 4.8 (0.890) | 5.5 (1.09) | 5 (0.771) | 0.7 (0.200) | 0.2 (0.064) |
Concentrations listed represent final PCB concentrations in cell culture media
Treatment compositions representing different products at various points in the dechlorination process (see also Table 1)
Treatment compositions representing dechlorination products at 10 and 24 h of dechlorination without the parent compound, PCB 77
Treatment composition representing the dechlorination products of 24 h dechlorination with the increased concentration of PCB 77 present at 10 h of dechlorination
Treatment composition representing the concentrations of PCB 77 present at 10 and 24 h of dechlorination
Cell culture
Primary endothelial cells (ECs) were isolated from porcine pulmonary arteries as described previously (Hennig et al. 1984). Cell culture media consisted of medium 199 (M199) containing 10 % fetal bovine serum (FBS). At 70–80 % confluence, cells were incubated overnight with treatment media (M199 with 1 % FBS), followed by exposure to PCB 77 or respective treatment mixtures for 24 h. Treatment mixtures were added to cell culture at a final concentration of 5 μM, which represented a maximum concentration of 0.1 %v/v DMSO/media (see Tables 1 and 2 for PCB treatment concentrations). Cell viability was tested in the presence of dechlorination products using the Vybrant MTT Cell Proliferation Assay Kit (Invitrogen, Eugene, OR) according to manufacturer guidelines.
Assessment of superoxide (O2−) levels
Superoxide levels were assessed as recently reported (Majkova et al. 2011). Cells were grown to confluence in four-chamber culture slides (BD Biosciences, Bedford, MA). After 4 h exposure to PCB treatments (5 μM total concentrations; Table 1), the cells were rinsed 2× with Krebs-Ringer buffer (KRB) and incubated with 5 μM dihydroethidium (DHE) or KRB (blank) at 37 °C for 30 min. Cells were rinsed with KRB, fixed with 10 % buffered formalin, and washed with PBS. Slides were mounted with ProLong Gold antifade reagent with DAPI for nuclei staining. Slides were evaluated with an Olympus BX61W1 fluorescence microscope. Mean fluorescence intensity was quantified using ImageJ 1.42q (NIH, Bethesda, MD).
Assessment of NFκB activation
Nuclear extracts were prepared from endothelial cells cultured as described above in 10-cm dishes and treated with PCB 77 or dechlorination mixtures for 4 h. The nuclear extraction was performed as described previously (Lim and Kim 2007). EMSA (electrophoretic mobility shift assay) of NFκB binding was kindly performed by Dr. Seong-Su Han of the University of Iowa, College of Medicine, using the DNA–protein binding detection kit (Gibco-BRL, Grand Island, NY) with radio-labeled oligonucleotides (Han et al. 2010c, 2012).
Measurement of CYP1A1, MCP-1, or VCAM-1 mRNA
CYP1A1, MCP-1, and VCAM-1 mRNA expression was assessed using real-time PCR (RT-PCR), as described previously (Majkova et al. 2009; Han et al. 2010a). Briefly, mRNA was isolated using TRIzol Reagent (Life Technologies, Carlsbad, CA) and quantified using a SmartSpec Plus Spectrophotometer (BIO-RAD, Philadelphia, PA). cDNA was then generated using Promega AMV reverse transcriptase (Fisher Scientific, Waltham, MA), and RT-PCR was performed using Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA) and the following porcine primer sequences: β-actin sense 5′-TCA TCA CCA TCG GCA ACG-3′ and antisense, 5′-TTC CTG ATG TCC ACG TCG-3′; CYP1A1 sense 5′-TGG AGA GGC AAG AGTAGT TGG-3′ and anti-sense 5′-GGC ACA ACG GAG TAG CTC ATA-3′; MCP-1 sense 5′-CGG CTG ATG AGC TAC AGA AGA GT-3′ and antisense, 5′-GCT TGG GTT CTG CAC AGA TCT-3′; and VCAM-1 sense 5′-TGG AAA GAC ATG GCT GCC TAT-3′ and antisense, 5′-ACA CCA CCC CAG TCA CCA TAT-3′. Assays were run using the Applied Biosystems 7300 real-time PCR System (Life Technologies, Carlsbad, CA) using absolute quantification. The raw data were quantified using a standard curve and analyzed with a manual CT threshold of 0.200. Sample data were normalized to individual β-actin values.
Measurement of CYP1A1, MCP-1, and VCAM-1 protein
CYP1A1 and VCAM-1 protein levels were assessed by Western blotting, as described previously (Venkatachalam et al. 2008). After semi-dry transfer, nitrocellulose membranes were incubated for 2 h in blocking buffer (5 % non-fat milk in Tris-buffered saline containing 0.05 % Tween 20). Rabbit polyclonal CYP1A1 (H-70) and VCAM1 (C-19)-R (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) primary antibodies were diluted 1:1,000 in blocking buffer and incubated overnight at 4 °C, and the secondary antibody was diluted 1:4,000. β-actin primary and secondary antibodies were diluted 1:10,000 (Majkova et al. 2009). MCP-1 protein levels were measured in cell culture media diluted 1:7 using an OptEIA Human MCP-1 ELISA Kit (BD Biosciences, San Diego, CA) according to manufacturer guidelines.
Statistical analysis
Values are reported as means±SEM obtained from a representative sample set of a minimum of three independent experiments, unless otherwise stated. Comparisons were made by one-way analysis of variance with Tukey’s test for post hoc analysis, using SigmaPlot 12.0 software (Systat Software, San Jose, CA). Statistical probability of pvalue <0.05 was considered significant. Different statistical marker letters indicate statistical differences.
Results
Dechlorination alters cellular oxidative stress and viability
PCB-induced cellular dysfunction is characterized by increased oxidative stress and subsequent activation of inflammatory pathways including NFκB. The uncoupling of CYP1A1 leads to the generation of reactive oxygen species (ROS), such as superoxide. Endothelial cell monolayers were exposed to representative dechlorination product mixtures (Table 1) for 4 h before ROS were visualized with DHE fluorescence (Fig. 1a). Strong staining was observed in cells exposed to 0 h (pure PCB 77) and 10 h dechlorination products, whereas mixtures representing longer periods of dechlorination showed decreased superoxide production. When DHE fluorescence was quantified and normalized to DAPI nuclear staining, fluorescence from initial dechlorination mixtures (0 and 10 h of dechlorination) indicated higher ROS production which decreased to control levels with further dechlorination (differences between groups approached significance, p=0.052; see Fig. 1b). A similar trend was observed with regard to viability, where no changes were observed in cells treated with PCB vehicle (DMSO) and the 48 h dechlorination product, although cell viability was decreased in 0 h (parent compound, PCB 77), 10 h and 24 h dechlorination product treatments to 69±1 %, 61±3 %, and 87±2 % of control, respectively.
Fig. 1.
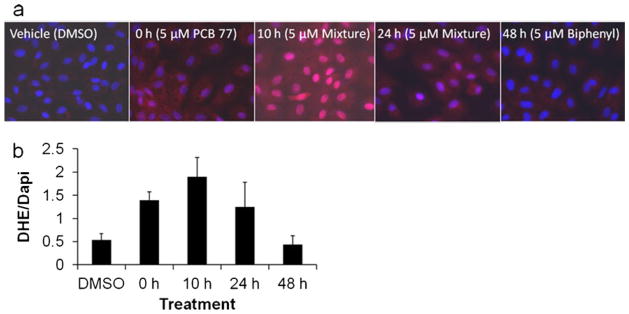
Dechlorination of PCB 77 alters cellular oxidative stress. Cells grown on chamber slides were treated with DMSO (vehicle control) or dechlorination products with a total concentration of 5 μM total concentrations and were incubated for 4 h. Cells were then treated with N, N′-(1,2-dihydroxyethylene) bisacrylamide (DHE, in red) for superoxide detection. Slides were fixed with DAPI (in blue) for nuclear staining; a fluorescent images were recorded at 40× and b DHE fluorescence was quantified and normalized to DAPI
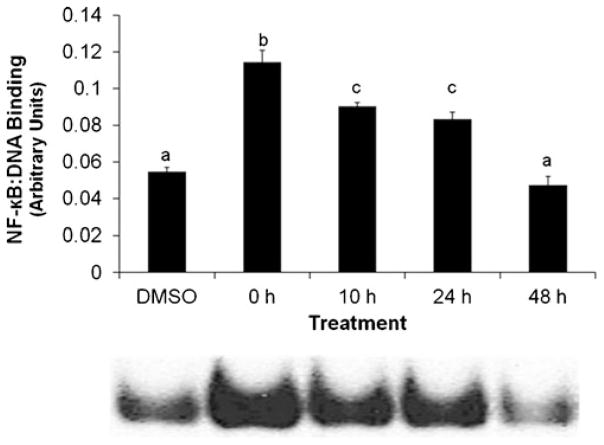
NFκB activation is attenuated by dechlorination
Data from an NFκB EMSA showed that cells treated for 4 h with the dechlorination products exhibited a prominent increase in NFκB activation when treated with the 0 h product, or pure PCB 77. Intermediate activation by the 10 and 24 h products was observed; NFκB/DNA binding for these treatments was significantly decreased compared with exposure to the 0 h product but remained increased relative to the vehicle control and biphenyl (48 h product; see Fig. 2). Biphenyl was consistently within control expression levels for superoxide fluorescence, viability, and NFκB/DNA binding. The 10 and 24 h dechlorination products were increased from control but did not exhibit a consistent linear change from parent PCB 77, 0 h of dechlorination, expression levels. We, therefore, tested a series of treatment mixtures to delineate between the effects of the dechlorination products and the residual PCB 77.
Fig. 2.
Dechlorination of PCB 77 attenuates NFκB activation. Cells were treated with vehicle control (DMSO), 0 (PCB 77, 5 μM) 10, 24, and 48 h dechlorination mixtures (5 μM) and incubated for 4 h. Both PCB 77 and dechlorination products (10 and 24 h product treatments) significantly increased DNA binding of NFκB over DMSO and biphenyl (48 h treatment). Results are given as mean±SEM. Different statistical marker letters represent significant differences among treatment groups (determined by Pvalue≤0.05)
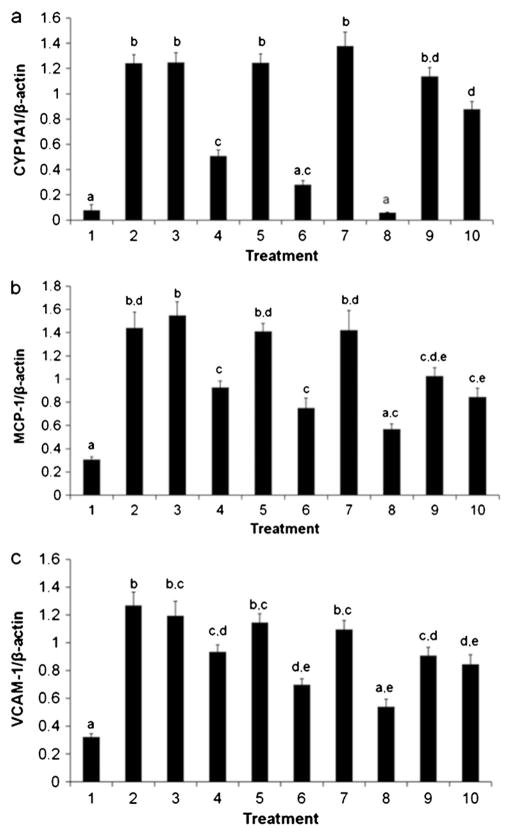
Dechlorination products alter CYP1A1, MCP-1, and VCAM-1 mRNA expression
CYP1A1, VCAM-1, and MCP-1 levels were assessed in mRNA using treatment groups 1–10 (Fig. 3, Table 2). CYP1A1 expression is a sensitive marker of coplanar PCB-mediated cellular activation and toxicity. Thus, significant response from all treatments containing PCB 77 was expected (Fig. 3a). Indeed, all treatments that contained pure or some PCB 77 (treatments 2, 3, 5, 7, 9, and 10) exhibited almost maximal CYP1A1 expression. There was no difference in CYP1A1 expression among the pure PCB 77 (treatment 2) and both 10 h (treatment 3) and 24 h (treatment 5) dechlorination mixtures. Interestingly, when 10 and 24 h dechlorination treatments lacked PCB 77 (treatments 4 and 6, respectively), induction of CYP1A1 was reduced linearly. Both MCP-1 and VCAM-1 mRNA expression patterns (Fig. 3b and c, respectively) followed approximately the CYP1A1 treatment patterns, suggesting some correlation between CYP1A1 induction and inflammatory markers in these dechlorination experiments. As with CYP1A1 expression, MCP-1 and VCAM-1 expression exhibited a linear decrease to control levels when treated with mixtures representing 10, 24, and 48 h dechlorination products without PCB 77 (treatments 4, 6, and 8), indicating that any byproduct contribution to the inflammatory response was eliminated by further dechlorination. Total dechlorination to biphenyl (48 h product) showed no statistical difference from control, indicating that it was relatively benign in our EC model. The presence of residual parent PCB dominated the inflammatory response in product mixtures, but mRNA expression levels of PCB 77 alone were moderately attenuated at the lower concentrations.
Fig. 3.
Dechlorination products alter CYP1A1 (a), MCP-1 (b), and VCAM-1 (c) mRNA expression. The presence of PCB 77 significantly upregulated mRNA inflammatory markers in ECs; inflammation decreased with further dechlorination of the parent compound. Please see Table 2 for detailed treatment descriptions. The vehicle, DMSO (treatment 1), was applied at 0.1 %v/v for all treatments. Treatments representing the dechlorination products at 0 (treatment 2), 10 (treatment 3), 24 (treatment 5), and 48 h (treatment 8) are the same as those tested previously for NFκB, superoxide production, and viability. Treatments 4 and 6 excluded PCB 77 while maintaining the representative concentrations of the dechlorination products found at 10 and 24 h, respectively. In contrast, treatments 9 and 10 contained only PCB 77 at the concentrations found in the 10 and 24 h mixtures, respectively. Treatment 7 consisted of the 24 h dechlorination mixture with the level of PCB 77 found in the 10 h mixture. Different statistical marker letters represent significant differences among treatment groups (determined by Pvalue≤0.05). For example, in CYP1A1, treatment 2 (statistical marker “b”) is significantly different from treatment 1 (“a”) and treatment 4 (“c”)
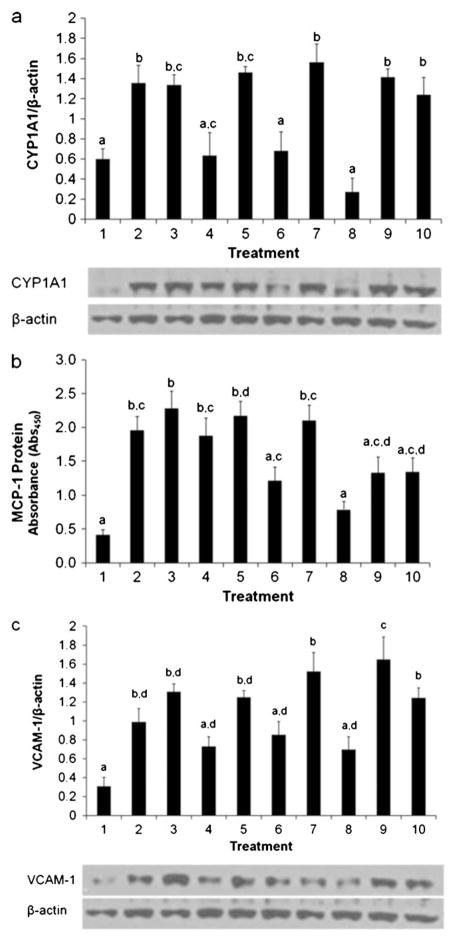
Degree of dechlorination affects CYP1A1, MCP-1, and VCAM-1 protein expression
Endothelial cells were treated with the dechlorination mixtures mentioned above (Table 2) in order to determine the effects of PCB 77 dechlorination on endothelial cell inflammatory response expressed in protein markers (Fig. 4). Treatments containing the parent compound showed strong correlations in both mRNA and protein. Similar to the mRNA expression levels, CYP1A1 protein levels were highest for treatments containing PCB 77 (Fig. 4a). However, treatments that did not contain PCB 77 showed a marked decrease in CYP1A1 and MCP-1 protein expression, correlated to the degree of dechlorination. Significant increases in MCP-1 expression were observed with the 0 h product (PCB 77, treatment 2) and, to a lesser degree, with dechlorination products containing decreasing concentrations of PCB 77 (treatments 3, 5, and 8). VCAM-1 protein expression of dechlorination product mixtures without PCB 77 (treatments 4 and 6) and biphenyl (treatment 8) were not significantly different from control. The presence of PCB 77 in all treatment groups corresponded to significant increases in VCAM-1 protein expression above control levels. These data suggest a complicated interplay between inflammatory parameters in response to PCB 77 dechlorination product exposure. Nevertheless, the presence of PCB 77 in the individual treatments markedly induced vascular inflammation.
Fig. 4.
Dechlorination mixtures alter CYP1A1 (a), MCP-1 (b), and VCAM-1 (c) protein expression. The presence of PCB 77 significantly upregulated protein inflammatory markers in ECs; inflammation decreased with further dechlorination of the parent compound. Treatments are the same as described in Fig. 3 (please see Table 2 for detailed treatment descriptions). Statistical differences among treatment groups (determined by Pvalue≤0.05) are represented by marker letters, as stated previously
Discussion
PCBs are man-made chemicals that were used in hundreds of industrial and commercial applications (Environmental Protection 2011) and are byproducts of certain industries today (Hu and Hornbuckle 2010). Even though their mass production has been banned, PCBs are chemically stable, persistent environmental toxicants that can cause harmful health effects. Remediation or detoxification of contaminated sites is difficult and expensive, and it is not clear to what extent these remediation processes have to occur before their disease potential is mitigated. The present study was designed to test the hypothesis that dechlorination of coplanar PCB 77 diminishes its pro-inflammatory potential in vascular endothelial cells. This is an important issue because PCBs can damage vascular tissues and thus contribute to cardiovascular diseases such as atherosclerosis. In fact, a recent study reported increased hospitalization rates for acute myocardial infarction and diabetes mellitus in populations residing near areas contaminated with persistent organic pollutants (Sergeev and Carpenter 2010a, b). Furthermore, the administration of PCB 77 to male apolipoprotein E (ApoE) −/− mice has been shown to promote atherosclerosis (Arsenescu et al. 2011).
The lining of blood vessels is protected by the endothelium, and endothelial cells play an active role in physiological processes such as regulation of vessel tone, blood coagulation, and vascular permeability. Dysfunction of endothelial cells is a critical underlying cause of the initiation of cardiovascular diseases such as atherosclerosis. Coplanar PCBs are initiators of endothelial dysfunction and exert their toxicity through binding to the AhR. AhR target genes, including CYP1A1, are considered a source of oxidative stress in endothelial cells and subsequent vascular dysfunction (Kopf et al. 2010; Kopf and Walker 2010). We confirmed in the present study the oxidative stress potential of coplanar PCBs. In fact, CYP1A1 induction was maximal in cells exposed to pure PCB 77 and remained elevated in all dechlorination mixtures that contained PCB 77, independent of its relative concentration. When residual amounts of PCB 77 were excluded from the dechlorination mixtures, CYP1A1 induction was markedly reduced, suggesting that the presence of the parent coplanar PCB is necessary for maximal CYP1A1 expression. Furthermore, we have previously shown that coplanar PCBs 77, 126, and 169 increased expression of the CYP1A1 gene, oxidative stress (DCF fluorescence), and the DNA-binding activity of NFκB (Hennig et al. 2002). In the current study, oxidative stress and DNA-binding of NFκB decreased with increasing dechlorination. In fact, when PCB 77 was completely dechlorinated to biphenyl, oxidative stress and NFκB levels approached control levels. This suggests that effective dechlorination should be as complete as possible to avoid oxidative stress-mediated tissue toxicity.
Oxidative stress-induced transcription factors such as NFκB, which regulate inflammatory cytokine and adhesion molecule production, play critical roles in the induction of inflammatory responses and subsequent atherosclerotic lesion formation. We have demonstrated previously that coplanar PCBs can cause endothelial cell dysfunction as determined by inflammatory markers, such as expression of inflammatory cytokines and adhesion molecules, i.e., MCP-1 and VCAM-1, respectively (Han et al. 2010b; Hennig et al. 2005; Majkova et al. 2009). In our analysis of the dechlorination product mixtures, we found that inflammatory patterns of MCP-1 and VCAM-1 were similar to CYP1A1 patterns induced by pure PCB 77 and the dechlorination mixtures that contained residual PCB 77, suggesting that the parent compound is necessary for an endothelial inflammatory response. The 10 h dechlorination byproducts also induced an MCP-1 and VCAM-1 response that was alleviated by further dechlorination. Again, as was found with CYP1A1, total dechlorination to biphenyl did not induce MCP-1 and VCAM-1 inflammatory parameters in our endothelial cell model system.
The dechlorination process decreased the parent PCB 77 but contributed to various lower-chlorinated PCBs. Furthermore, the ratio and proportion of these lower-chlorinated PCBs changes with degree of dechlorination. Even though their level of interaction with the AhR is not well characterized, lower-chlorinated PCBs may contribute to cellular dysfunction. For example, PCB 15, which was found in relatively high concentrations in the dechlorination mixtures, can contribute to liver carcinogenesis (Espandiari et al. 2003). PCB 11, another common PCB in our dechlorination mixtures, recently has been discovered in relatively large amounts in commercial paint pigments (Hu and Hornbuckle 2010). Furthermore, other lower chlorinated PCBs generated during dechlorination have been shown to be metabolically activated by electrophilic quinoid species, which can bind to DNA (Oakley et al. 1996). Our data suggest that the temporary formation of lower chlorinated PCBs during dechlorination can contribute in part to induction of inflammatory parameters.
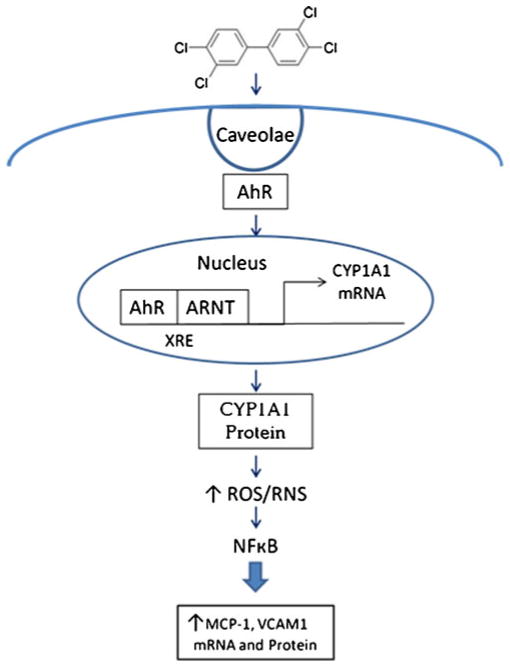
In addition, the lipophilic nature of PCBs enables them to perturb membrane structures. We have evidence that membrane domains like caveolae are critically involved in endothelial inflammation induced by exposure to PCBs. For example, PCB 77 can accumulate in caveolae-rich fractions of endothelial cells (Lim et al. 2008), and upregulation of endothelial MCP-1 by PCB 77 is caveolin-1-dependent (Majkova et al. 2009). Evidence from our laboratory implicates caveolae as a regulatory platform involved in endothelial activation and inflammation by environmental contaminants (Fig. 5). As a regulatory platform, caveolae facilitate the interaction of PCBs with the AhR, which in turn promotes the upregulation of CYP1A1, a phase I-detoxifying enzyme. CYP1A1 metabolism of coplanar PCBs, like PCB 77, is inefficient, leading to protein uncoupling and release of superoxide, which promotes the formation of ROS in the cell (Schlezinger et al. 2006). The cell responds to this change in redox status by activating the transcription factor NFκB, which regulates enzymes and inflammatory parameters associated with endothelial dysfunction and cell injury such as MCP1 and VCAM1 (de Winther et al. 2005). Further studies are needed to understand whether caveolae have differential selectivity for various PCB species such as those present in dechlorination mixtures and to determine if the interaction of these congeners with caveolae has potential to induce endothelial cell dysfunction.
Fig. 5.
PCB 77 increases ROS production and downstream cellular dysfunction. PCB 77 interacts with caveolae in the plasma membrane, is endocytosed into the cell, and interacts with the AhR and its chaperone ARNT (aryl hydrocarbon receptor nuclear translocater), leading to nuclear translocation, XRE (xenobiotic response element) binding and CYP1A1 upregulation. CYP1A1 protein uncoupling occurs during PCB 77 metabolism and leads to an increase in cellular ROS. NFκB is activated in response to the change in cellular redox status and translocates to the nucleus where it acts as a transcription factor to upregulate adhesion molecules and cytokines
In summary, our study provides evidence that dechlorination of highly chlorinated PCBs is beneficial for protecting the vasculature from oxidative stress-induced inflammation and subsequent pathologies like atherosclerosis. Highly chlorinated PCBs are persistent and cytotoxic and a significant risk factor to endothelial injury and associated vasculature pathologies. The pro-inflammatory potential of the dechlorination mixtures that we observed appears to depend largely on the presence of the parent compound (e.g., PCB 77). Clearly, cytotoxicity decreased relative to degree of dechlorination, and the final dechlorination product, biphenyl, was nontoxic in our endothelial cell model system. More studies are needed to understand threshold concentrations of dechlorination mixtures that are required to prevent or mitigate compromised health associated with exposure to environmentally toxic chlorinated biphenyls.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Environmental Health Sciences grant P42ES007380, American Recovery and Reinvestment Act (ARRA) funds (3P42ES007380-13S1), and with funds from the University of Kentucky Agricultural Experiment Station. We would like to thank Christopher R. Barton1 and Alex N. Palumbo1 for their contributions to the preliminary work on this project. Thanks also to Dr. Seong-Su Han for his work with the NFκB binding assay. A special thanks to Michael Petriello for his editorial assistance.
Footnotes
Undergraduate ARRA summer research student
Responsible editor: Leif Kronberg
Contributor Information
Katryn Eske, University of Kentucky SRP Center, University of Kentucky, Lexington, KY 40536-0200, USA. Graduate Center for Nutritional Sciences, University of Kentucky, Kentucky SRP Center, Room 599, Wethington Building, 900 South Limestone Street, Lexington, KY 40536-0200, USA.
Bradley Newsome, University of Kentucky SRP Center, University of Kentucky, Lexington, KY 40536-0200, USA. Department of Chemistry, University of Kentucky, Lexington, KY 40506-0055, USA.
Sung Gu Han, University of Kentucky SRP Center, University of Kentucky, Lexington, KY 40536-0200, USA. Department of Food Science of Animal Resources, College of Animal Bioscience and Technology, Konkuk University, Seoul 143-701, Korea.
Margaret Murphy, University of Kentucky SRP Center, University of Kentucky, Lexington, KY 40536-0200, USA. Graduate Center for Nutritional Sciences, University of Kentucky, Kentucky SRP Center, Room 599, Wethington Building, 900 South Limestone Street, Lexington, KY 40536-0200, USA.
Dibakar Bhattacharyya, University of Kentucky SRP Center, University of Kentucky, Lexington, KY 40536-0200, USA. Department of Chemical and Materials Engineering, University of Kentucky, Lexington, KY 40506-0046, USA.
Bernhard Hennig, Email: bhennig@uky.edu, University of Kentucky SRP Center, University of Kentucky, Lexington, KY 40536-0200, USA.
References
- Arsenescu V, et al. Polychlorinated biphenyl 77 augments angiotensin II-induced atherosclerosis and abdominal aortic aneurysms in male apolipoprotein E deficient mice. Toxicol Appl Pharmacol. 2011;257(1):148–154. doi: 10.1016/j.taap.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol. 2009;201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Brown JF, Feng H, Bedard DL, Brennan MJ, Carnahan JC, May RJ. Environmental dechlorination of PCBs. Environ Toxicol Chem. 1987;6(8):579–593. doi: 10.1002/etc.5620060802. [DOI] [Google Scholar]
- de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25(5):904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- Doskey PV, Andren AW. Concentrations of airborne PCBs over Lake Michigan. J Great Lakes Res. 1981;7(1):15–20. doi: 10.1016/S0380-1330(81)72018-5. [DOI] [Google Scholar]
- Environmental Protection Agency. [Accessed 12 Nov 2012];Cleanup and disposal of polychlorinated biphenyls (PCBs) 2011 http://www.epa.gov/region2/cleanup/pcb/
- Environmental Protection Agency. [Accessed 19 Oct 2012];Hazardous Waste Regulations. 2012 http://www.epa.gov/epawaste/laws-regs/regs-haz.htm.
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol. 2003;186(1):55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Ganey PE, Boyd SA. An approach to evaluation of the effect of bioremediation on biological activity of environmental contaminants: dechlorination of polychlorinated biphenyls. Environ Health Perspect. 2005;113(2):180–185. doi: 10.1289/ehp.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010a;246(1–2):74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010b;246(1–2):74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol. 2012 doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, et al. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Molecular cancer. 2010c;9:97. doi: 10.1186/1476-4598-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawari J, Demeter A, Samson R. Sensitized photolysis of polychlorobiphenyls in alkaline 2-propanol: dechlorination of Aroclor 1254 in soil samples by solar radiation. Environ Sci Technol. 1992;26(10):2022–2027. doi: 10.1021/es00034a022. [DOI] [Google Scholar]
- Hennig B, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181(3):174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Oesterling E, Toborek M. Environmental toxicity, nutrition, and gene interactions in the development of atherosclerosis. Nutr Metab Cardiovasc Dis. 2007;17(2):162–169. doi: 10.1016/j.numecd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect. 2005;113(1):83–87. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4(5):489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol. 2010;44(8):2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537–546. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol. 2010;245(1):91–99. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Kim CW. Functional characterization of the promoter region of the chicken elongation factor-2 gene. Gene. 2007;386(1–2):183–190. doi: 10.1016/j.gene.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Lim EJ, et al. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176(2–3):71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol Appl Pharmacol. 2011;251(1):41–49. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237 (1):1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley GG, Robertson LW, Gupta RC. Analysis of polychlorinated biphenyl-DNA adducts by 32P-postlabeling. Carcinogenesis. 1996;17(1):109–114. doi: 10.1093/carcin/17.1.109. [DOI] [PubMed] [Google Scholar]
- Puga A, Sartor MA, Huang MY, Kerzee JK, Wei YD, Tomlinson CR, Baxter CS, Medvedovic M. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385–404. doi: 10.1385/ct:4:4:385. [DOI] [PubMed] [Google Scholar]
- Rodenburg LA, Du S, Lui H, Guo J, Oseagulu N, Fennell DE. Evidence for dechlorination of polychlorinated biphenyls and polychlorinated dibenzo-p-dioxins and -Furans in wastewater collection systems in the New York Metropolitan Area. Environ Sci Technol. 2012;46(12):6612–6620. doi: 10.1021/es300560q. [DOI] [PubMed] [Google Scholar]
- Rodenburg LA, Guo J, Du S, Cavallo GJ. Evidence for unique and ubiquitous environmental sources of 3,3′-dichlorobiphenyl (PCB 11) Environ Sci Technol. 2010;44(8):2816–2821. doi: 10.1021/es901155h. [DOI] [PubMed] [Google Scholar]
- Ruzo LO, Zabik MJ, Schuetz RD. Photochemistry of bioactive compounds. Photoproducts and kinetics of polychorinated biphenyls. J Agri Food Chem. 1974;22(2):199–202. doi: 10.1021/jf60192a045. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77(4):422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sergeev AV, Carpenter DO. Exposure to persistent organic pollutants increases hospitalization rates for myocardial infarction with comorbid hypertension. Prim Prev Insights. 2010a;2:1–9. doi: 10.4137/PPRI.S4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev AV, Carpenter DO. Residential proximity to environmental sources of persistent organic pollutants and first-time hospitalizations for myocardial infarction with comorbid diabetes mellitus: a 12-year population-based study. Int J Occup Med Environ health. 2010b;23(1):5–13. doi: 10.2478/v10001-010-0010-y. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Arzuaga X, Chopra N, Gavalas VG, Xu J, Bhattacharyya D, Hennig B, Bachas LG. Reductive dechlorination of 3,3′,4,4′-tetrachlorobiphenyl (PCB77) using palladium or palladium/iron nanoparticles and assessment of the reduction in toxic potency in vascular endothelial cells. J Hazard Mater. 2008;159(2–3):483–491. doi: 10.1016/j.jhazmat.2008.02.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran EM, Bhattacharyya D, Bachas LG. Development of reactive Pd/Fe bimetallic nanotubes for dechlorination reactions. J Mater Chem. 2011;21(28):10454–10462. doi: 10.1039/c1jm11435b. [DOI] [PMC free article] [PubMed] [Google Scholar]