Abstract
Objective
MAP0004 is an investigational product which delivers dihydroergotamine (DHE) through the lung via a breath-synchronized metered dose inhaler. The objective of this study was to compare the acute effects of orally inhaled and intravenous (IV) DHE to placebo on maximum change and area under the curve for pulmonary arterial systolic pressure (PASP).
Research design and methods
A randomized, double-blind, placebo-controlled, 3-period, crossover study of 24 health adults. Trial registration NCT01089062. Study assessments included pharmacokinetics, electrocardiograms (ECG), and validated echocardiographic (Doppler)-derived measures of PASP by echocardiogram. The primary endpoint was the absolute change in calculated PASP using area under the curve, 0 to 2 hours (AUC0–2h).
Results
The change in PASP with IV DHE was significantly different than MAP0004 and placebo (AUC0–2h2857, 2624, and 2453 mmHg*min, respectively). After a second dose of MAP0004, AUC0–4h remained lower with MAP0004 than with a single dose of IV DHE. Adverse events were more common with IV DHE than with MAP0004 or placebo. None of the treatments produced clinically significant changes in PASP or other cardiac parameters. Changes in PASP were significantly smaller with MAP0004 compared with IV DHE.
Conclusion
These results indicate the effects 1 mg of orally inhaled DHE on the cardiovascular system are less than with 1 mg of IV DHE, and that serial echocardiography can be a useful noninvasive means of assessing acute systemic effects.
Keywords: dihydroergotamine, intravenous, MAP0004, echocardiogram, echo, inhaled, pulmonary artery systolic pressure
Introduction
Assessing the acute pharmacological effects of an inhaled medication with vasoactive properties is important as absorption through the lung may be rapid, with the potential to result in clinically significant effects on blood pressure and cardiac function. One of the key parameters measured with vasoactive drugs is pulmonary arterial systolic pressure (PASP), as it reflects preload, a critical determination of cardiac function. Traditionally, PASP has been measured via indwelling catheter, which is an accurate but invasive methodology.
Dihydroergotamine (DHE) mesylate has been used for the acute treatment of migraine for over 60 years with demonstrated efficacy, rapid onset, low recurrence rates, and long lasting effect. However, subcutaneous, intranasal, and rectal formulations have erratic absorption and unpredictable plasma concentrations, and many patients srequire administration of DHE in a health care setting. MAP0004 is an investigational product which delivers DHE through the lung via a breath-synchronized metered dose inhaler and demonstrates rapid pulmonary absorption and a consistent pharmacokinetic (PK) profile in studies of healthy volunteers.1–4 After single doses, oral inhalation of MAP0004 resulted in rapid absorption of DHE, with a median time to peak plasma concentration (Tmax) that ranged from 7–11 minutes.2–4 In addition, mean peak plasma concentrations (Cmax) were approximately 2500 pg/mL with MAP0004 1.0 mg, while Cmax was at least 10-fold greater following administration of intravenous (IV) DHE 1.0 mg.3,4
In randomized, placebo-controlled clinical trials, MAP0004 was effective for the acute treatment of migraine.5,6 These trials reported no evidence of clinically relevant effects on blood pressure or changes in the electrocardiogram (ECG) following a single dose of MAP0004. However, since absorption of DHE through the lungs is rapid and DHE has vasoactive properties, there is a theoretical concern about high cardiac concentrations following inhalation, with the potential for cardiac vasoactive effects. Therefore, a study was undertaken to evaluate and compare the acute effects of IV DHE and orally inhaled MAP0004 on PASP as assessed by echocardiography and other parameters of cardiac function. Echocardiography was chosen instead of right heart catheterization, as echocardiography can be repeated on multiple days in the same subject.
Methods
Our study was a randomized, double-blind, placebo-controlled, 3-period crossover study to compare the effects of IV DHE and orally inhaled MAP0004 on PASP and cardiac function. The study was conducted in compliance with the Declaration of Helsinki, in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, and in accordance with the US Food and Drug Administration regulations for informed consent and protection of patients’ rights. The protocol, amendments, and informed consent forms were reviewed and approved by an appropriate Institutional Review Board, Copernicus Group IRB, Research Triangle Park, NC, USA. Each participant was required to provide written informed consent prior to enrollment in the study.
Subject selection
Healthy nonsmoking adult men and women aged 18–45 years were eligible if they had normal cardiac function as determined by a medical history and physical examination, echocardiogram, and 12-lead ECG. A normal echocardiogram was defined as one showing no significant structural, functional, or hemodynamic abnormalities. In addition, minimal echocardiography image quality was required as defined by the presence of fewer than two nonvisualized myocardial segments and a measurable tricuspid regurgitant jet.7,8 A normal or non-clinically significant ECG obtained at screening after ≥5 minutes of rest in the supine position was defined as heart rate ≥40 and ≤100 beats/min (resting) and a Fridericia-corrected QT (QTcF) interval duration <430 milliseconds for males and <450 milliseconds for females. Women were required to practice adequate contraception or to be surgically sterile.
Subjects were excluded for the following reasons: any contraindication to DHE; use of any excluded medication within 10 days; history of hemiplegic or basilar migraine; diagnosis of cancer within 5 years; major surgery, vascular surgery or sepsis within 6 months; or unstable psychiatric illness requiring medication or hospitalization within the previous 12 months. Women who were pregnant, breastfeeding, or planned to become pregnant were also excluded. Any subject with clinically significant hepatic, renal, pulmonary, cardiac or other illness that could interfere with the conduct of the study was excluded. Also excluded was any subject with hypertension (systolic blood pressure [BP] >140 mmHg or diastolic BP >90 mmHg) or any subject with two of the following four risk factors: receiving antihypertensive medication for hypertension; hyperlipidemia defined as low density lipoprotein >159 mg/dL or high density lipoprotein <40 mg/dL or on prescribed treatment; family history of coronary artery disease; or diabetes mellitus.
Study treatments
Eligible subjects were randomized in a 2:1 ratio of females:males, which is similar to the ratio in migraine sufferers. Each subject received all three treatments in random order over three visits separated by intervals of 1 week, as described in Table 1. Each subject received both inhaled and IV treatments with drug or placebo at each visit. The second inhaled administration occurred 2 hours after the first dose. The subject, treating physician, sponsor, and site staff performing study assessments were blinded to treatment assignment. MAP0004 inhaler and placebo inhaler were supplied in identical packages, and IV DHE and IV placebo were prepared by a pharmacist not otherwise involved with the study. MAP0004 was delivered via an inhaler, which used a breath-triggered, synchronized mechanism that provided significantly increased drug delivery compared with conventional pressurized metered-dose inhalers. The inhaler delivered 0.5 mg DHE per actuation. MAP0004 was manufactured under the direction of MAP Pharmaceuticals, (Mountain View, CA, USA).
Table 1.
Study treatments and assessments
| Treatment A | Treatment B | Treatment C | |
|---|---|---|---|
| First dose | Inhaler placebo IV DHE 1.0 mg | MAP0004 1.0 mg nominal dose IV placebo | Inhaler placebo IV placebo |
| 2 hours of study assessments: PK sampling, echo, 12-lead ECG, vital signs | |||
| Second dose (2 hours after first dose) | Inhaler placebo | MAP0004 1.0 mg nominal dose | Inhaler placebo |
| 2 hours of study assessments: PK sampling, echo, 12-lead ECG, vital signs | |||
Abbreviations: DHE, dihydroergotamine; IV, intravenous; PK, pharmacokinetics; ECG, electrocardiography.
Study assessments
An echocardiogram was obtained using a Phillips i33 machine (Koninklijke Philips Electronics NV, Amsterdam, Netherlands) by a certified sonographer who was experienced in performing research echocardiography and was blinded to the study treatments. A full echocardiogram was obtained 30 minutes before and at 30 and 90 minutes after each dose of study medication. An abbreviated echocardiogram for assessing PASP was obtained at 3, 5, 7, 10, 12, 15, 20, 25, 30, 60, 90 and 120 minutes after each dose. In each case, at least three consecutive cardiac cycles were acquired for each measurement.
Pulmonary artery pressure was estimated in an independent core laboratory using the peak velocity of the tricuspid valve regurgitant jet, using the simplified Bernoulli equation and combining this value with an estimate of the right atrial pressure from inferior vena cava diameter and respiratory changes per American Society of Echocardiography guidelines.9 A technologist blinded to study treatment performed preliminary quantitation on all images. A cardiologist with level three credentials in echocardiography and blinded to study treatment reviewed and approved all measurements and assessments. Intra and interobserver variability testing was completed prior to performing any study measurements and showed excellent reproducibility with an intraclass correlation coefficient of 0.96 for estimated right ventricular pressure with an error rate of 0.06.
An ECG was recorded using a GE ECG machine (GE Healthcare Bio-Sciences Corp, Piscataway, NJ, USA) 30 minutes before each dose and then recorded continuously for 2 hours after each dose of study medication. The ECG was printed for analysis at 30 minutes before each dose and at 1, 9, 14, 29, 59, and 119 minutes after each dose.
Safety was assessed from adverse events (AE), vital signs (temperature, pulse, respiratory rate, and BP), laboratory measurements (chemistry, hematology, and urinalysis), echocardiography, and ECG. Subjects remained as inpatients for at least 2 hours after the final observation.
A blood sample was obtained at 30 minutes before each dose and at 2, 10, 15, 30, 60, and 120 minutes after each dose of study medication for determination of DHE and 8′ hydroxyl-dihydroergotamine (OH-DHE) plasma concentrations using an electrospray multiple reaction monitoring liquid chromatography–mass spectrometry. PK parameters were calculated, including Cmax, Tmax, and area under the curve (AUC), as determined by a trapezoidal method from time 0 to 2 hours post first dose (AUC0–2h), and the sum of AUC0–2h post first dose and AUC0–2h post second dose (AUC0–4h).
Statistical analysis
The pharmacodynamic population included all randomized subjects who received at least one dose of study drug and completed at least one pharmacodynamic assessment. The safety population included all randomized subjects who received at least one dose of the study drug. The PK population included all randomized subjects who received at least one dose of the study drug and provided PK samples for at least one treatment period.
The primary endpoint was absolute change in PASP, which was evaluated with AUC0–2h. Secondary endpoints were the proportion of subjects with an increase in PASP >10 mmHg at 2 hours; maximum change from baseline in PASP at 2 hours; absolute change in PASP over time from the start of the first dose to 2 hours after the second dose (AUC0–4h); changes in BP over the 2 hour dosing period after each dose; and change in corrected QT (QTc) intervals from baseline to 14 minutes after the first and second doses of study medication.
Absolute change over time and maximum change from baseline in PASP were analyzed using an analysis of variance (ANOVA) model for a 3-period crossover trial. For each endpoint, pairwise comparisons between MAP0004 and IV DHE, between MAP0004 and placebo, and between IV DHE and placebo were tested. For endpoints expressed as proportions, McNemar’s test was used for comparisons between MAP0004 and IV DHE, between MAP0004 and placebo, and between IV DHE and placebo. In these analyses, only data from the two treatments being compared were utilized.
Results
Twenty-four healthy subjects were randomized and received the study drug; six subjects discontinued before completing all study assessments, five for consent withdrawn, and one for an AE (see Table 5). Valid pharmacodynamic data were available for 20 subjects. Baseline demographic characteristics are shown in Table 2.
Table 5.
Summary of treatment emergent adverse events (TEAE)
| Number (%) of subjects
|
|||
|---|---|---|---|
| IV DHE 1.0 mg (n = 24) | MAP0004 1.0 mg (n = 24) | Placebo (n = 24) | |
| Reporting at least one | |||
| TEAE | 19 (79.2) | 10 (41.7) | 6 (25.0) |
| Study drug related | 19 (79.2) | 6 (25.0) | 4 (16.7) |
| TEAE | |||
| TEAE leading to discontinuation | 1 (4.2) | 0 | 0 |
| Serious TEAE | 0 | 0 | 0 |
| TEAE by severity | |||
| Mild | 12 (63.2) | 9 (90.0) | 6 (100.0) |
| Moderate | 7 (36.8) | 1 (10.0) | 0 |
| Severe | 0 | 0 | 0 |
| IV DHE 1.0 mg (n = 20) | MAP0004 1.0 mg (n = 20) | Placebo (n = 20) | |
|
| |||
| TEAE occurring at least twice | |||
| Headache | 6 (30.0) | 5 (25.0) | 1 (5.0) |
| Nausea | 10 (50.0) | 1 (5.0) | 0 |
| Feeling hot | 8 (40.0) | 0 | 0 |
| Pharmaceutical product complaint (taste) | 4 (20.0) | 2 (10.0) | 1 (5.0) |
| Dizziness | 3 (15.0) | 2 (10.0) | 1 (5.0) |
| Burning sensation | 4 (20.0) | 0 | 0 |
| Paresthesia | 3 (15.0) | 0 | 0 |
| Flushing | 2 (10.0) | 0 | 1 (5.0) |
| Chest discomfort | 2 (10.0) | 0 | 0 |
| Head discomfort | 2 (10.0) | 0 | 0 |
| Vomiting | 2 (10.0) | 0 | 0 |
Abbreviations: TEAE, treatment emergent adverse events; DHE, dihydroergotamine; IV, intravenous.
Table 2.
Baseline demographic characteristics of all randomized subjects
| Characteristic | N = 24 |
|---|---|
| Mean (SD) age, years | 26.6 (6.6) |
| Range | 19.9–40.1 |
| Male:female | 8:16 |
| Mean (SD) weight, kg | 81.8 (17.0) |
| Mean (SD) BMI, kg/m2 | 28.2 (4.8) |
| Ethnicity and race, n (%) | |
| Hispanic or Latino | 1 (4.2) |
| Black or African American | 1 (4.2) |
| American Indian or Alaska Native | 10 (41.6) |
| White or Caucasian | 12 (50.0) |
Abbreviations: SD, standard deviation; BMI, body mass index.
Pharmacodynamic assessment
For the primary endpoint PASP AUC0–2h, no significant difference was observed between MAP0004 and placebo (P = 0.194) (Table 3). However, mean PASP AUC0–2h was significantly higher with IV DHE compared with placebo (P = 0.001) and MAP0004 (P = 0.025). Mean PASP AUC0–4h, was significantly higher (P = 0.001) with IV DHE versus placebo; no significant differences were observed between other groups. For the period following the second dose of MAP0004 (2–4 hours), mean PASP AUC2–4h was significantly higher with IV DHE versus placebo (P = 0.002) and for MAP0004 versus placebo (P = 0.040). No significant difference was observed between IV DHE and MAP0004 (P = 0.201). The mean maximum change in PASP0–2h was significantly higher with IV DHE and MAP0004 compared with placebo (P = 0.001 and P = 0.031, respectively). One subject in the IV DHE group had an increase in PASP >10 mmHg*min from baseline to 2 hours after the first dose (P < 0.0001 versus MAP0004 and placebo, McNemar’s test).
Table 3.
Summary of changes in PASP following treatment with IV DHE, MAP0004, and placebo
| Parameter | Mean (standard deviation) PASP
|
||
|---|---|---|---|
| IV DHE 1.0 mg (n = 20) | MAP0004 1.0 mg (n = 20) | Placebo (n = 20) | |
| AUC0–2h (mmHg*min) | 2856.9 (510.78) | 2624.0 (392.10) | 2453.0 (404.76) |
| P-valuea | 0.001 versus placebo | 0.194 versus placebo | 0.025 versus MAP0004 |
| AUC0–4h (mmHg*min) | 5700.1 (1016.86) | 5336.4 (823.51) | 4907.0 (773.11) |
| P-valuea | 0.001 versus placebo | 0.069 versus placebo | 0.056 versus MAP0004 |
| AUC2–4h (mmHg*min) | 2843.2 (529.15) | 2712.4 (455.26) | 2454.0 (389.41) |
| P-valuea | 0.002 versus placebo | 0.040 versus placebo | 0.201 versus MAP0004 |
| IV DHE 1.0 mg (n = 24) | MAP0004 1.0 mg (n = 24) | Placebo (n = 24) | |
|
| |||
| Maximum change in PASP (mmHg) 0–2 hours | 7.8 (2.4) | 6.1 (2.8) | 4.0 (1.7) |
| P-valuea | 0.001 versus placebo | 0.031 versus placebo | 0.108 versus MAP0004 |
Note:
Calculated using the ANOVA model for a 3-period crossover.
Abbreviations: AUC, area under the curve; ANOVA, analysis of variance; DHE, dihydroergotamine; IV, intravenous; PASP, pulmonary arterial systolic pressure.
No significant ECG changes were observed at 14 minutes (time closest to the expected Tmax) after the first or second dose of MAP0004. One subject in the placebo group had a QTc interval above 450 milliseconds (increase from 432 milliseconds at baseline to 452 milliseconds at 14 minutes after the second placebo dose). No subjects had a change from baseline in QTc interval >30 milliseconds or >60 milliseconds after the first or second dose of study medication. No subjects had a PR interval increase of 25% from baseline and >200 milliseconds or a QRS interval increase of 25% from baseline and >100 milliseconds.
Mean changes in systolic BP and diastolic BP were 6–12 mmHg with IV DHE at 15 minutes after the dose. For MAP0004, mean changes in systolic BP were 3–5 mmHg and mean changes in diastolic BP were 1–2 mmHg. At 15 minutes after the second dose of MAP0004, mean changes in systolic BP and diastolic BP were <2 mmHg and approximated those of the placebo group.
Pharmacokinetics
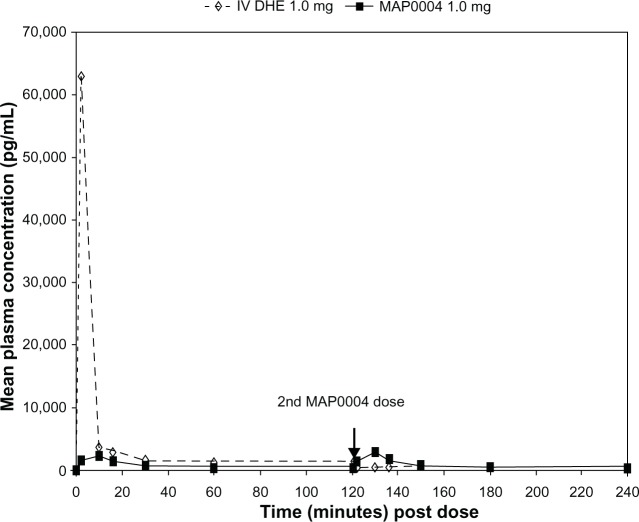
Peak plasma DHE concentrations were >20-fold higher after IV than after inhaled administration of DHE (Table 4), while Tmax was less than 10 minutes for both treatments. Mean Cmax and Tmax for MAP0004 were similar after the first and second doses. Plasma concentrations of 8′ OH-DHE were markedly lower than the parent compound after both IV and inhaled administration (Table 4). Over the entire 4 hour dosing interval that included 2 doses of MAP0004, mean plasma DHE and 8′ OH-DHE concentrations remained below levels achieved with IV DHE, except for the brief period immediately following the second dose of MAP0004 (Figure 1).
Table 4.
Pharmacokinetic parameters for DHE and 8′ OH-DHE
| Treatment | Mean (standard deviation)
|
|||
|---|---|---|---|---|
| Cmax (pg/mL) | Tmax (minutes) | AUC0–2h (pg*hour/mL) | AUC0–4h (pg*hour/mL) | |
| DHE | ||||
| IV DHE 1.0 mg, n = 18 | 58321.0 (9157.41) | 3.1 (0.24) | 8018.5 (1503.52) | 9020.1 (1708.87) |
| MAP0004 1.0 mg (first dose), n = 19 | 2474.7 (755.52) | 10.7 (3.00) | 1318.9 (492.51) | – |
| MAP0004 1.0 mg (second dose), n = 19 | 3063.0 (1351.30) | 9.2 (2.55) | 3041.6a,b (1237.48) | |
| 8′ OH-DHE | ||||
| IV DHE 1.0 mg, n = 17 | 328.6 (145.95) | 8.5 (4.33) | 214.2 (80.41) | 311.8 (129.54) |
| MAP0004 1.0 mg (first dose), n = 16 | 97.9 (37.78) | 19.4 (6.55) | 104.9 (40.68) | – |
| MAP0004 1.0 mg (second dose), n = 18 | 117.9 (51.40) | 15.1 (6.39) | 154.9 (81.90) | 277.9a,c (116.99) |
Notes:
AUC0–4h is the sum of AUC0–2h for the first and second doses;
n = 18;
n = 15; Subjects with values below the limit of quantitation were excluded.
Abbreviations: AUC, area under the curve; DHE, dihydroergotamine; IV, intravenous; OH-DHE, hydroxy-dihydroergotamine; Cmax, mean peak plasma concentration; Tmax, median time to peak plasma concentration.
Figure 1.
Mean plasma DHE concentrations over time with IV DHE 1.0 mg and MAP0004 1.0 mg.
Abbreviations: DHE, dihydroergotamine; IV, intravenous.
Safety and tolerability
At least one AE was reported in 19 subjects with IV DHE, ten with MAP0004, and six with placebo. Eight AEs, seven in the IV DHE group and one in the MAP0004 group, were of moderate intensity, and 27 were of mild intensity. The most common AEs were headache, nausea, and feeling hot with IV DHE, and headache and dizziness with MAP0004 (Table 5). Ten (50%) subjects experienced nausea with IV DHE, one (5%) subject with MAP0004, and none with placebo. One AE in the IV DHE group led to study discontinuation for flushing, headache, and nausea. No clinically significant changes in laboratory values, vital signs, or other changes in cardiac function were observed in any subject.
Discussion
This study was undertaken because of potential concerns that migraine patients could experience greater exposure to the cardiovascular system from DHE when delivered via oral inhalation versus the IV route. The results from this study showed that MAP0004 and placebo exhibited relatively comparable effects on PASP. In contrast, PASP AUC0–2h was significantly higher in the IV DHE group than in both MAP0004 and placebo groups. However, for PASP AUC0–4h, IV DHE was significantly higher than the placebo group, but no significant difference was observed between MAP0004 and either IV DHE or placebo groups. These results indicate that the effect of orally inhaled MAP0004 on the CV system is less than that observed with IV DHE and comparable to placebo.
A unique design feature of this study was the use of serial echocardiography measurements to assess PASP. One advantage of echocardiography over pulmonary artery catheter monitoring is greater subject acceptability for repeated use in a study such as this, which used a 3-period crossover design requiring frequent and repeated measurements of PASP. The primary endpoint – AUC0–2h for PASP – was an average of the results from frequent echocardiography measurements obtained over 2 hours, and reproducibility of Doppler measurements of PASP was excellent. This composite endpoint and other quality control measures successfully reduced the variability that previously has been reported with Doppler echocardiography assessment in patients with pulmonary disease.10–14 The ability to repeat the 4-hour-long sessions on three separate occasions makes serial echocardiography a useful methodology for evaluating vasoactive properties of inhaled medications.
The findings of this study are strengthened by the use of a double-dummy design. The double-dummy design ensured that all study personnel were blinded to study treatment, limited the potential for bias, and enhanced the objectivity of the findings, particularly the AE reported. Another design feature of the study is that it included a second dose of MAP0004 given 2 hours after the first dose to evaluate plasma concentration and the effect on PASP with repetitive doses. Nevertheless, the effect on PASP was comparable between the first and second dose of MAP0004 and remained less than that observed with a single dose of IV DHE. The results from this study utilizing a second dose provide some support for the safety of repeat administration of MAP0004 during and acute episode of migraine. IV DHE was used in this study because it was administered in previous comparative studies with MAP0004.2–4
The effects of MAP0004 after the first dose on systolic and diastolic BP were lower than those observed with IV DHE, and the effects of MAP0004 after the second dose were similar to those observed with placebo. Clinical studies of episodic migraine patients observed no effect of MAP0004 on BP after single doses.5,6 The observed lack of an effect of MAP0004 on BP provides further evidence of the absence of a clinically relevant effect on the CV system. In addition, no significant changes in ECG parameters (QTc, PR, QRS) were observed with MAP0004 at 14 minutes after both the first and second dose.
In this study, the Cmax of DHE was approximately 20-fold higher after IV DHE than after inhaled MAP0004; the Cmax of the 8′ OH-DHE metabolite was also markedly lower compared with the parent DHE. PK parameters for the 8′ OH-DHE metabolite previously were reported.1,2 The DHE Cmax observed with MAP0004 in this study was consistent with the DHE Cmax observed in other studies of healthy subjects and acute migraine patients, which also found a 10- to 20-fold difference in Cmax compared with IV DHE.2–4 The Cmax observed in this study compares favorably with that reported for the intranasal formulation (1,040 pg/mL).15 These investigators also studied the PKs of DHE in subjects during and outside of a migraine episode, finding no difference.
MAP0004 exhibited a good safety and tolerability profile, with primarily mild drug-related AEs and no serious AEs reported. Most of the reported AEs were in the IV DHE group including one AE of headache and nausea that led to study discontinuation. The incidence of nausea was lower with MAP0004 than with IV DHE, which is consistent with findings from early Phase I studies2–4 and with the low rate of nausea reported in efficacy studies of MAP0004.5,6 In addition, the improved tolerability may be related to the substantially lower peak plasma levels with MAP0004 versus IV DHE.1 The low rate of nausea also supports findings from receptor binding studies, which revealed differences in binding affinity following IV and inhaled delivery of DHE for receptors traditionally associated with the AEs of DHE.16
In summary, the results from this study provide evidence of the CV safety of orally inhaled MAP0004. Based on the results of this study, the effects of orally inhaled MAP0004 on cardiac circulation appear to be less than those with IV DHE, and thus MAP0004 may be a desirable alternative for the acute treatment of migraine.
Acknowledgments
The authors would like to acknowledge the editorial assistance of Richard S Perry (PharmD) in the preparation of this manuscript, which was supported by MAP Pharmaceuticals, Inc, Mountain View, CA, USA. This study was funded by MAP Pharmaceuticals, Inc, Mountain View, CA, USA.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. RJN is a consultant for Piramal Clinical Research and within the past year has received compensation from Piramal. PSD performed this work as part of an institutional research contract between Duke Clinical Research Institute and MAP Pharmaceuticals, under which she received no financial benefit. SCC has nothing to disclose. BM has nothing to disclose. DJK and SK are employees of MAP Pharmaceuticals. A portion of this data was presented as a poster at the American Society for Clinical Pharmacology and Therapeutics 112th Annual Meeting, March 3, 2011.
References
- 1.Shrewsbury SB, Cook RO, Taylor G, Edwards C, Ramadan NM. Safety and pharmacokinetics of dihydroergotamine mesylate administered via a Novel (Tempo) inhaler. Headache. 2008;48(3):355–367. doi: 10.1111/j.1526-4610.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 2.Shrewsbury SB, Kori S, Miller SD, Pedinoff A, Weinstein S. Randomized, double blind, placebo controlled study of the safety, tolerability and pharmacokinetics of MAP0004 (orally-inhaled DHE) in adult asthmatics. Curr Med Res Opin. 2008;24(7):1977–1985. doi: 10.1185/03007990802160677. [DOI] [PubMed] [Google Scholar]
- 3.Forst A, Febbraro S, Kellerman D, et al. An open-label, two-period crossover study comparing the pharmacokinetics and tolerability of LEVADEX™ (MAP0004, orally inhaled DHE) and intravenous DHE (DHE45®) in smoking and non-smoking adult volunteers. Headache. 2011;51(Suppl 1):17. Abstract P31. [Google Scholar]
- 4.Kellerman D, Kori S, Forst A, et al. Lack of drug interaction between the migraine drug MAP0004 (orally inhaled dihydroergotamine) and a CYP3A4 inhibitor in humans. Cephalalgia. 2012 Jan;32(2):150–158. doi: 10.1177/0333102411432299. [DOI] [PubMed] [Google Scholar]
- 5.Aurora SK, Rozen TD, Kori S, Shrewsbury SB. A randomized, double blind, placebo controlled study of MAP0004 in adult patients with migraine. Headache. 2009;49(6):826–837. doi: 10.1111/j.1526-4610.2009.01453.x. [DOI] [PubMed] [Google Scholar]
- 6.Aurora SK, Silberstein SD, Kori SH, et al. MAP0004, orally inhaled DHE: a randomized, controlled study in the acute treatment of migraine. Headache. 2011;51(4):507–517. doi: 10.1111/j.1526-4610.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- 7.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Milan A, Magnino C, Veglio F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr. 2010;23(3):225–239. doi: 10.1016/j.echo.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Fisher MR, Criner GJ, Fishman AP, et al. NETT Research Group Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J. 2007;30(5):914–921. doi: 10.1183/09031936.00033007. [DOI] [PubMed] [Google Scholar]
- 11.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naeije R, Torbicki A. More on the non-invasive diagnosis of pulmonary hypertension: Doppler echocardiography revisited. Eur Respir J. 1995;8(9):1445–1449. [PubMed] [Google Scholar]
- 13.Tramarin R, Torbicki A, Marchandise B, Laaban JP, Morpurgo M. Doppler echocardiographic evaluation of pulmonary artery pressure in chronic obstructive pulmonary disease. A European multicentre study. Working Group on Noninvasive Evaluation of Pulmonary Artery Pressure. European Office of the World Health Organization, Copenhagen. Eur Heart J. 1991;12(2):103–111. doi: 10.1093/oxfordjournals.eurheartj.a059855. [DOI] [PubMed] [Google Scholar]
- 14.Subhedar NV, Shaw NJ. Intraobserver variation in Doppler ultrasound assessment of pulmonary artery pressure. Arch Dis Child Fetal Neonatal Ed. 1996;75(1):F59–F61. doi: 10.1136/fn.75.1.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert H, Cabiac MD, Dubray C, Lavène D. Human pharmacokinetics of dihydroergotamine administered by nasal spray. Clin Pharmacol Ther. 1996;60(3):265–275. doi: 10.1016/S0009-9236(96)90053-3. [DOI] [PubMed] [Google Scholar]
- 16.Cook RO, Shrewsbury SB, Ramadan NM. Reduced adverse event profile of orally inhaled DHE (MAP0004) vs IV DHE: potential mechanism. Headache. 2009;49(10):1423–1434. doi: 10.1111/j.1526-4610.2009.01510.x. [DOI] [PubMed] [Google Scholar]