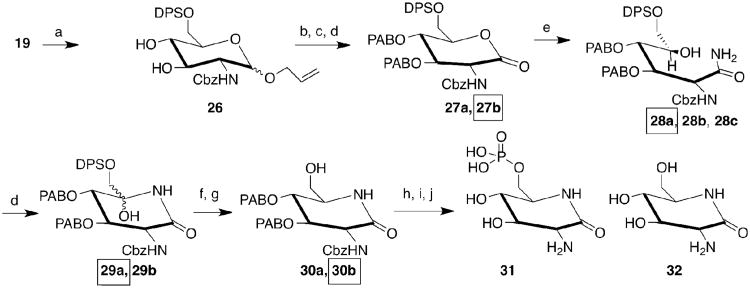
Scheme 2. 2-Amino-glucono-1,5-lactam-6-phosphate Synthesis.
a) DPS-Cl, py; b) PAB-Cl, BaO, Ba(OH)2•8H2O, DMF, then PAB-I, KOtBu, toluene; c) SeO2, AcOH, dioxane, reflux; d) Dess-Martin reagent, py; e) 7N NH3 in MeOH; f) HCOOH, NaBH3CN, CH3CN, reflux; g) TBAF, THF, AcOH; h) POCl3, py, 30m 0°C, then H2O; i) CHCl3, H2O, DDQ, 80°C; j) Pd(OH)2/C, NH4•HCOO, H2O/MeOH, reflux. PAB = p-N-(pivaloyl)aminobenzyl; DPS = tert-Butyldiphenylsilyl. A box indicates the major product component from each step; only the major component was carried through to the next step.