Figure 4.
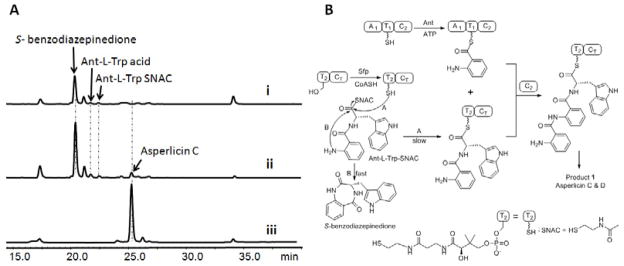
The first module of AspA iteratively utilizes two molecules of Ant. (A) The dipeptidyl Ant-L-Trp-SNAC (Gao, et al., 2012) is a surrogate substrate for M2 of AspA: the holo form of M1 (50 μM A1-T1-C2) was preloaded with Ant (1 mM Ant, 3 mM ATP) for 1 hour while in parallel the T2-CT didomain was converted to the holo (HS-pantetheinyl) form using 20 μM Sfp and 1mM CoASH. The two solutions were mixed with addition of 400 μM Ant-L-Trp-SNAC and incubated overnight before aliquots were analyzed by LC/MS (280 nm). Trace i shows the spontaneous products (S-benzodiazepinedione and Ant-L-Trp acid) formed from an assay without T2CT di-domain; trace ii shows products formed from an assay including holo T2CT. In addition to the spontaneously formed products, formation of asperlicin C can be detected. Trace iii shows the profile of products formed from full length AspA starting from Ant, L-Trp and ATP. (B) Reaction scheme: M1 loaded covalently with Ant on the pantetheinyl arm of T1 reacts with Ant-L-Trp-S-pantetheinyl-T2-CT to yield the tripeptidyl Ant-Ant-L-Trp-S-T2-CT form of the bi-domain T2-CT protein fragment. That can be acted on by CT to yield asperlicin C, D and 1. The added Ant-L-Trp- SNAC is proposed to undergo acyl exchange onto the HS-pantetheinyl arm of T2-CT in competition with hydrolysis to Ant-L-Trp and intramolecular cyclization to S-benzodiazepinedione.
