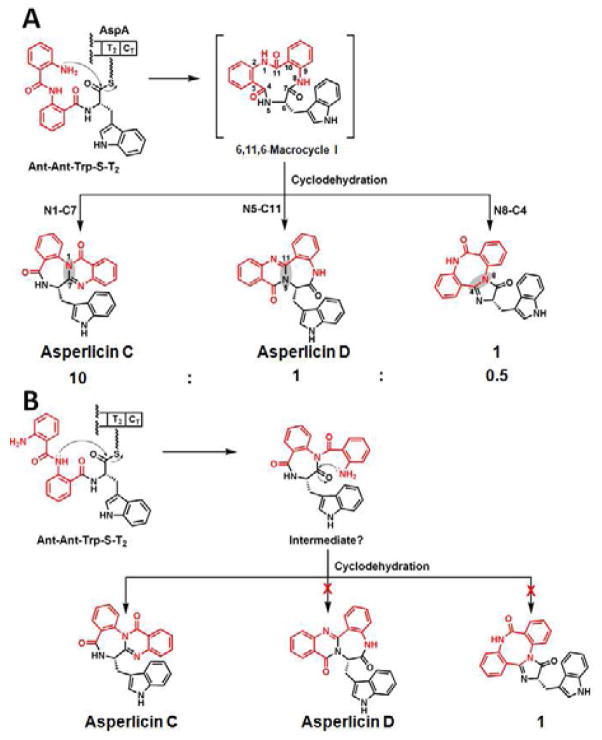
Figure 6.
Mechanistic proposal for generation of a constant ratio of asperlicin C (major) and asperlicin D (minor) from macrocyclizing release of a linear Ant-Ant-LTrp-S-T2 thioester by the CT domain. (A) The attack of the free NH2 of the Ant1 residue generates a transient 6,11,6-tricyclic product macrocycle I. Subsequent transannular attack and dehydration can proceed with distinct regiochemistries of intramolecular amide capture of carbonyl groups: N1 on C7=O gives major product asperlicin C, N5 on C11=O gives asperlicin D, while N8 on C4=O could give a third asperlicin regioisomer, putative product 1. Whether these steps happen in one of the active sites of AspA or in solution have not yet been determined. (B) Alternative mode of tripeptidyl chain release by initial attack of the amide NH of Ant2 residue on the thioester carbonyl would yield a diketopiperazine as nascent product. That can go on to asperlicin C as shown but has the wrong connectivity to generate the observed minor product asperlicin D and 1 and so is ruled out.