Table 3.
Nucleophile scope in benzoxathiole coupling.
| entry | substrate | nucleophile | product | yield (%) |
|---|---|---|---|---|
| 1 |
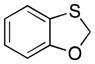 32 |
|
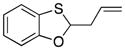 33 |
79 |
| 2a |
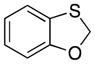 32 |
23 |
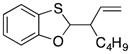 35 |
72 dr = 3.2:1 |
| 3a |
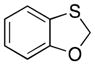 32 |
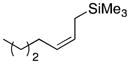 23-(cis) |
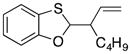 35′ |
66 dr = 1:4.6 |
| 4 |
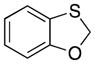 32 |
26 |
36 |
55 |
| 5 |
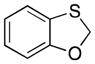 32 |
 28 |
37 |
65 |
| 6 |
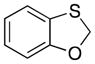 32 |
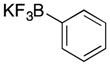 30 |
– | – |
| 7 |
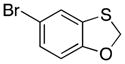 38 |
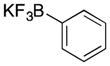 30 |
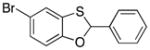 39 |
30 |
35 and 35′ are diastereomers. The relative stereochemical orientations have not been assigned.
