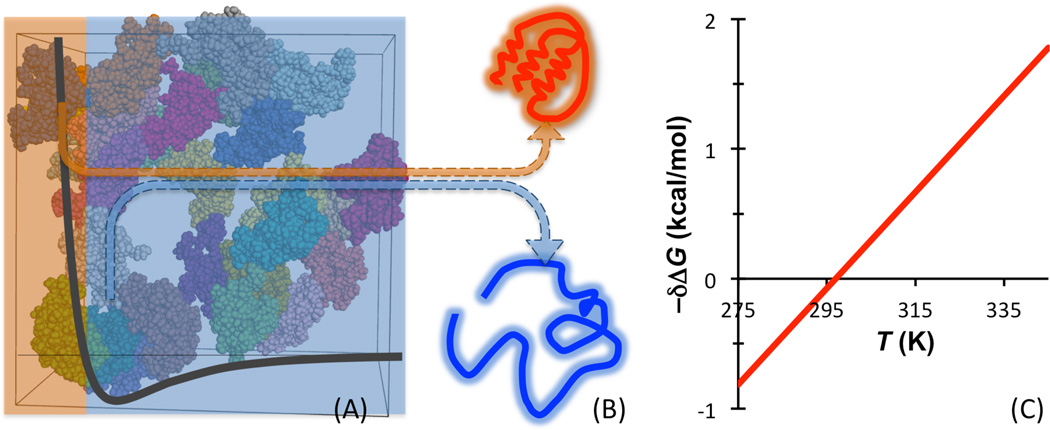
Fig. 2.
Illustration of the effects of macromolecular crowding on protein folding stability. (A) The interactions between a protein and surrounding crowder molecules consist of hard-core repulsion and longer-ranged attraction. (B) The hard-core repulsion leads to an entropic component that favors the folded state of the protein, whereas the longer-ranged attraction leads to an enthalpic component that favors the unfolded state. (C) The net effect of macromolecular crowding has a crossover temperature, where δΔG = 0.