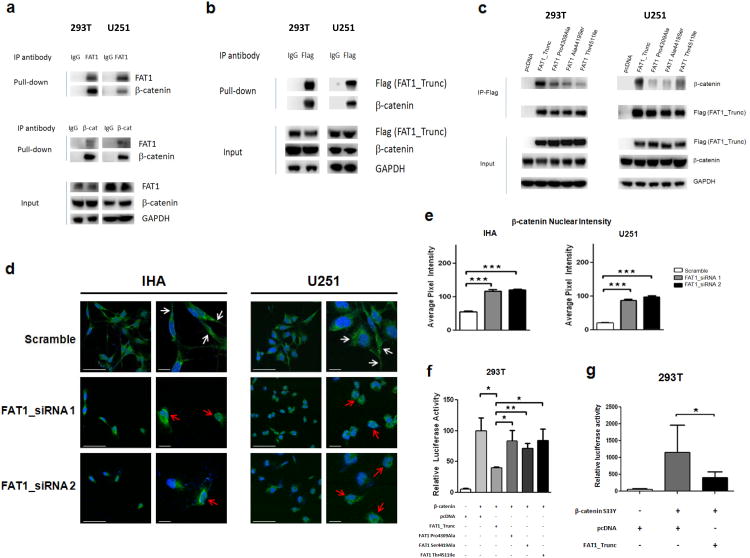
Figure 4. FAT1 is a β-catenin binding partner, inactivation of which causes aberrant Wnt pathway activation, translocation of β-catenin to the nucleus, and enhanced β-catenin-mediated transcription.
(a) Immunoprecipitation assays in indicated FAT1-expressing cell lines demonstrate that endogenous FAT1 binds endogenous β-catenin (top), and vice versa (middle). Antibody or IgG negative control as indicated.
(b) Immunoprecipitation assays demonstrate binding of transfected FAT1_Trunc to endogenous β-catenin.
(c) Immunoprecipitation of FLAG-tagged FAT1 constructs show loss of β-catenin binding in mutated FAT1.
(d) Translocation of β-catenin to the nucleus after FAT1 knockdown in GBM cells and immortalized human astrocytes (IHA). Cells were treated with indicated siRNAs; 48 hours later, cells were stained with DAPI (blue) and antibody against β-catenin (green). Representative photos from 3 independent repeat experiments, at lower and higher magnification. White arrows denote plasma membrane localization; red arrows, nuclear/perinuclear localization. Scale barsrepresent 50 μm.
(e) Quantification of results from (d). Nuclear staining expressed as pixel intensity, demonstrating increased nuclear localization of β-catenin after FAT1 knockdown.
(f) TOPFLASH reporter assay demonstrates reduction in β-catenin-dependent transcription following expression of non-mutated FAT1, but not FAT1 mutant constructs, in 293T cells. Reporter assays were performed as previously described52, and luciferase activity reported as relative fluorescence units for TOPFLASH, divided by fluorescence activity for the control (FOPFLASH). Error bars represent 1 standard deviation.
(g) Luciferase assay shows substantially increased β-catenin-mediated transcription after transfection with constitutively active β-catenin S33Y mutant, repressed with co-transfection of FAT1.
*p<.05, **p<.01, ***p<.001, t-test and ANOVA.