Abstract
The multidrug resistance-associated protein1 (MRP1/ABCC1) is a member of the ABCC transporter subfamily that mediates the efflux of pharmaceuticals, xenobiotics and steroid hormones, typically as glutathione, glucuronide or sulfate conjugates. Since loss of one transporter can be compensated by increasing the expression of other transporters and conjugation enzymes, we sought to examine compensatory changes in phase I, II and III enzyme expression in extrahepatic tissues, including the kidney, lungs and small intestine of intact or castrated Mrp1−/− male mice. In the kidney, the expression of several P450s, sulfotransferase 1a1 (Sult), glucuronosyltransferases (Ugt) and Mrps2–4, were significantly changed owing to castration alone. The only time genotype mattered was between the castrated FVB and Mrp1 knockout mice. In contrast, expression of the Ugts, Sult 1a1 and Mrp3 in the lungs was significantly downregulated in the Mrp1 knockout mice, so based exclusively on genotype. In the small intestine, there were interactions between steroid hormone levels and genotype, as the expression differences were only found in mice lacking Mrp1, and were changed between intact and castrated animals. The mechanism behind this pattern of expression may be to due to Nrf2 regulation, as its expression mirrors that of the phase II and phase III enzymes. These results indicate that compensatory responses owing to the loss of Mrp1 vary dramatically, depending on the particular tissue. This information will aid in the understanding of how drug uptake, disposition and elimination can be influenced by both hormone status and the presence and magnitude of transporter expression.
Keywords: multidrug resistance-associated protein 1, ABC transporter, sulfotransferase, glucuronosyltransferase, Nrf2, hormonal regulation, compensation
Introduction
The multidrug resistance-associated protein 1 (MRP1 or ABCC1) transports a variety of glucuronide-, glutathione- and sulfate-conjugated pharmaceuticals, conjugated steroid hormones, chemotherapy drugs, heavy metals, toxins and pesticides across the cell membranes (reviewed in Bakos and Homolya, 2007; Deeley and Cole, 2006). MRP1 is expressed ubiquitously in most tissues on the basolateral side of polarized cells (Peng et al., 1999; Stride et al., 1996). Other members of this subfamily include MRP2, which is found mainly in the liver, kidney and intestine on the apical side of cells (Paulusma et al., 1996), MRP3, which is found mainly in the liver, pancreas, kidney and intestine on the basolateral side (Belinsky et al., 1998; Cherrington et al., 2002; Kool et al., 1997), and MRP4, which is ubiquitously expressed in tissues on the apical side of cells (Kruh et al., 2001; Lee et al., 1998). These transporters are responsible for the efflux and ultimate elimination of a large number of pharmaceutical and other xenobiotics, and their presence and magnitude of expression can regulate drug disposition (reviewed in Toyoda et al., 2008; Xia et al., 2007; Zhou et al., 2008).
When the expression of a MRP transporter is reduced, there can be compensatory responses by other transporters and/or phase I and II detoxification enzymes. For example, rats deficient in Mrp2 compensate by inducing hepatic Mrp3 and renal Mrp4, which helps to divert the elimination of bilirubin from the liver to the kidney (Akita et al., 2001; Chen et al., 2005; Ogawa et al., 2000). In mice lacking Mrp2, there is conflicting transporter compensation data, with one group only showing an increase of renal Mrp4, but not of hepatic Mrp3 (Chu et al., 2006), while a second group demonstrated a modest (~60%) induction of Mrp3 in the same knockout mouse strain (Nezasa et al., 2006). Mice lacking Mrp1 show an induction of Mrp2 and Mrp5 in their livers (Bain and Feldman, 2003), while mice lacking Mrp3 show no hepatic induction of other transporters (Kitamura et al., 2010).
As with induction of transporters, compensatory induction of phase I and phase II enzymes, or their cofactors, owing to loss of an ABC transporter has also been demonstrated. For example, there are increases in glutathione levels in the majority of the organs (et al., 1997), decreases in activity of sulfotransferases, and increases in glucuronosyltransferase activity in the livers of mice lacking Mrp1 (Bain and Feldman, 2003). In Mrp2-deficient TR-rats, Ugt1a expression is increased by 3.5- and 5.5-fold, respectively, in their livers and kidneys (Johnson et al., 2005).
Beyond compensatory responses, many of the phase II enzymes and phase III transporters are expressed in a sexually dimorphic manner in which there is typically higher expression in female mice. For example, Sult1a1 expression is higher in the liver and kidney of females (Alnouti and Klaassen, 2006), and hepatic Ugt1a1 and renal Ugt1a2 expression is also higher in female mice (Buckley and Klaassen, 2007). Some of the phase III transporters also display sex differences, with higher Mrp3 and Mrp4 levels in the kidneys of females and higher hepatic Mrp1 and Mrp4 expression in female mice (Maher et al., 2005a).
Knowledge of how compensatory responses and hormones can alter phase II and phase III enzyme profiles in a tissue-specific manner is important, because these changes may alter the pharmacokinetic profile and toxicity of many pharmaceuticals and toxicants. Detoxification enzyme profiles and the influence of hormones on transporter expression have not been well characterized in extrahepatic tissues. Previous work has shown that Mrp1-deficient mice had a 5-fold reduction in serum testosterone levels compared with wildtype mice (Sivils et al., 2010), so we were interested in understanding whether the mechanism responsible for changes in detoxification enzyme expression in these mice is altered by reduced androgen levels.
The goals of this study were to: (1) examine three extrahepatic tissues that play a role in toxicant elimination: the kidney, lungs and small intestine, to determine if there are changes in the expression of phase I, II and III enzymes in response to the loss of Mrp1; and (2) because many of these enzymes are expressed in a sex-selective manner, we sought to determine whether testosterone levels influence their expression.
Materials and Methods
FVB and FVB/mrp1−/− mice
Eight FVB and FVB/mrp 1−/− mice were purchased from Taconic (Germantown, NY, USA), with half of the mice from each strain being castrated, while the other half were sham castrated. Animals were housed at 25 °C and 50% humidity, and provided water and food ad libitum. At 9 weeks of age, mice were euthanized with a CO2 overdose. The lungs, intestine and kidneys were divided into two sections: one was placed into TRI–Reagent (Sigma, St Louis, MO, USA) and stored at −80 °C for extraction of total RNA and the other frozen at −80 °C for cytosol and microsome preparation.
Testosterone Hydroxylation Assay
P450 enzymes regio- and steriospecifically hydroxylate testosterone, so the testosterone hydroxylation assay was used to measure the activity of multiple P450s simultaneously. Individual tissues were homogenized in cytosol buffer (10 mM HEPES, pH 7.4, 1 mM EDTA, 10% glycerol, 2 µg ml−1 each of aprotinin, leupeptin, and pepstatin), microsomes prepared as previously described (Bain and Feldman, 2003), and stored at −80 °C. Protein concentrations were determined using Bio-Rad’s protein dye (Hercules, CA, USA).
Microsomal protein (100–200 µg) was incubated in 0.1 M phosphate buffer (pH 7.4) with 40 nmol [14C] testosterone (53.6 mCi mmol−1; Amersham, Piscataway, NJ, USA) along with 1 mM NADPH for 10 min at 37 °C in a shaking water bath. To terminate the reaction and extract the hydroxylated products, 2 ml ethyl acetate was added twice, evaporated under nitrogen, resuspended in ethyl acetate and spotted on TLC plates. The plates were developed in 80% methylene chloride–20% acetone, again in 70% chloroform–17.5% ethyl acetate–12.5% ethanol, and then exposed to BioMax MR autoradiography film (Fisher Scientific, Pittsburg, PA, USA). Using the film as a template, the metabolites were cut from the TLC plate and quantitated by liquid scintillation (Baldwin and LeBlanc, 1992). Activity was measured as pmol metabolite produced per minute per milligram protein and converted into a percentage of total metabolites.
qPCR
Individual tissue samples were homogenized in Tri Reagent to recover total RNA, treated with RNase-free DNase (Promega, Madison, WI, USA), and their concentration determined by spectrophotometry. cDNA was prepared from RNA (2 µg), and quantitative polymerase chain reaction (qPCR) was used to determine the expression of selected phase II and phase III genes and Nrf2, using gene-specific primers (Table 1). A standard curve was constructed for each gene to determine the number of molecules. All samples (n = 3) were run in triplicate using Sybr Green (Qiagen, Valencia, CA, USA), and the number of molecules detected was normalized against Gapdh as a housekeeper. Each run was repeated twice.
Table 1.
qPCR primer sets
| Primer | Forward 5′ to 3′ | Reverse 5′ to 3′ | Tm (°C) |
|---|---|---|---|
| Mrp2 | atggtcctagacagcggcaagatt | ttcacactttcaatgccggcttcc | 60 |
| Mrp3 | agaaccaagcatcaaggtcccaga | aaggttctcaccaaagccctcaga | 60 |
| Mrp4 | caaagctgcaagccacatcctcat | agaccaaattgaggcctcggagaa | 60 |
| Ugt1a | gaaattgctgaggctttgggcaga | agagtgtgtgatgaatgcccgagt | 60 |
| Ugt2b | agttgagacaatgggccaag | gttgggtgaggaaactccaa | 61 |
| Sult1a1 | gcactttgatgcccactatgccaa | ttgccttggttcccagtatagcca | 60 |
| Sult1b1 | tggcaaggatgttgctgtctccta | ccagatattcttcccaggtgcca | 60 |
| Nrf2 | tcacacgagatgagcttagggcaa | tacagttctgggcggcgactttat | 60 |
| Gapdh | gccttccgtgttcctacc | gcctgcttcaccaccttc | 51 |
Statistical Analysis
Statistical differences (P ≤ 0.05) were determined using ANOVA followed by Tukey’s, or by Student’s t-test using Prism GraphPad software (La Jolla, CA, USA).
Results
Phase I Activity is Influenced by Both Castration and the Loss of Mrp1
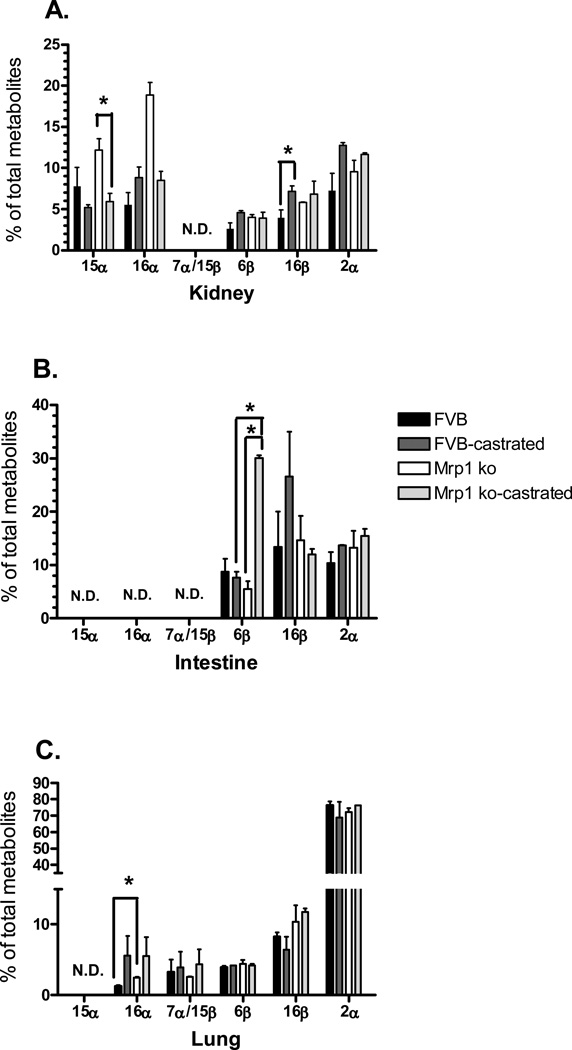
The activity of multiple cytochrome P450s was assessed in the kidneys, lungs and intestines of intact and castrated FVB and FVB/Mrp1−/− mice using testosterone as a substrate. The 15α-hydroxy testosterone product (Cypa4/5) was only detected in the kidney, and was significantly downregulated by castration (Fig. 1A). Likewise, 16β-OH testosterone (Cyp2b1, 2d1) formation in the kidney was also affected only by castration. The intestinal samples displayed a more complex patterning for 6β-hydroxylation (Cyp 3a, 2c), in which there were significant differences between genotypes and a significant increase owing to castration, but only in the mice lacking Mrp1 (Fig. 1B). In the lungs, 16α-OH (Cyp2b, 2c) testosterone formation was increased by 2-fold in the Mrp1 knockout animals compared with the wildtype (Fig. 1C) In the kidney, there was a similar pattern of 2.2-fold increased expression based upon genotype, but this was not quite statistically significant (P = 0.08).
Figure 1.
Cytochrome P450 activity. The hydroxylation activity of multiple P450 isoforms towards testosterone was determined (n = 3) for the kidney (A), small intestine (B) and lung (C). Values are expressed as the mean pmol min−1 mg−1 protein ± SD. Statistical differences (*) were determined by ANOVA followed by Tukey’s test (P < 0.05). ND, not detected.
Phase II Enzyme Expression
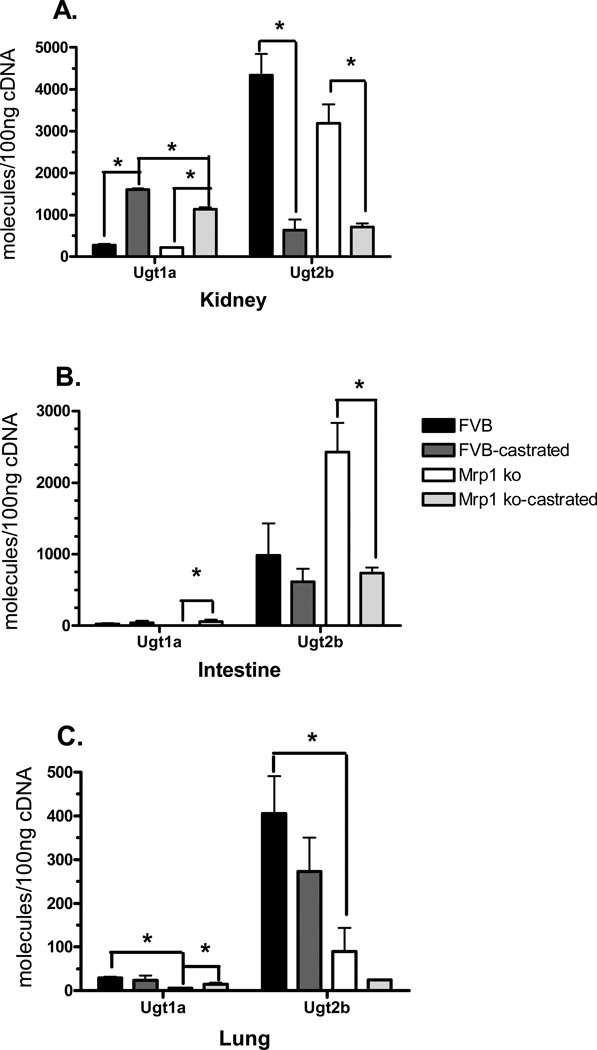
Examining the expression of Ugt1a and 2b mRNA in the kidneys revealed that transcript levels in this organ were predominantly dependent upon hormone status. Castration increased the levels of Ugt1a by 5.1- to 5.9-fold, and decreased the levels of Ugt2b by 4.5- to 6.8-fold in the kidneys from mice of both genotypes (Fig. 2A). In the intestine, Ugt expression was also dependent upon hormone status, but only for the mice lacking Mrp1. Ugt1a expression was increased in castrated Mrp1 knockout mice by 35-fold and Ugt2b expression was decreased by 3.8-fold (Fig. 2B). In the lungs, genotype played the predominant role in determining Ugt expression. In Mrp1 knockout mice, both Ugt1a and Ugt2b were downregulated by 4.8- and 4.5-fold, respectively (Fig. 2C). Castration of the Mrp1 knockout mice did increase Ugt1a transcript levels in the lungs, but the magnitude was much less than that found in the kidneys and intestines (Fig. 2C).
Figure 2.
Ugt mRNA expression in the kidney, small intestine, and lung. mRNA levels of Ugt1a and Ugt2b were determined by qPCR in the kidney (A), small intestine (B) and lung (C). Samples (n = 3) were run in triplicate and repeated twice. Data are expressed as copy number per 100 ng cDNA ± SD normalized to Gapdh. Statistical differences (*) were determined by ANOVA followed by Tukey’s test (p < 0.05).
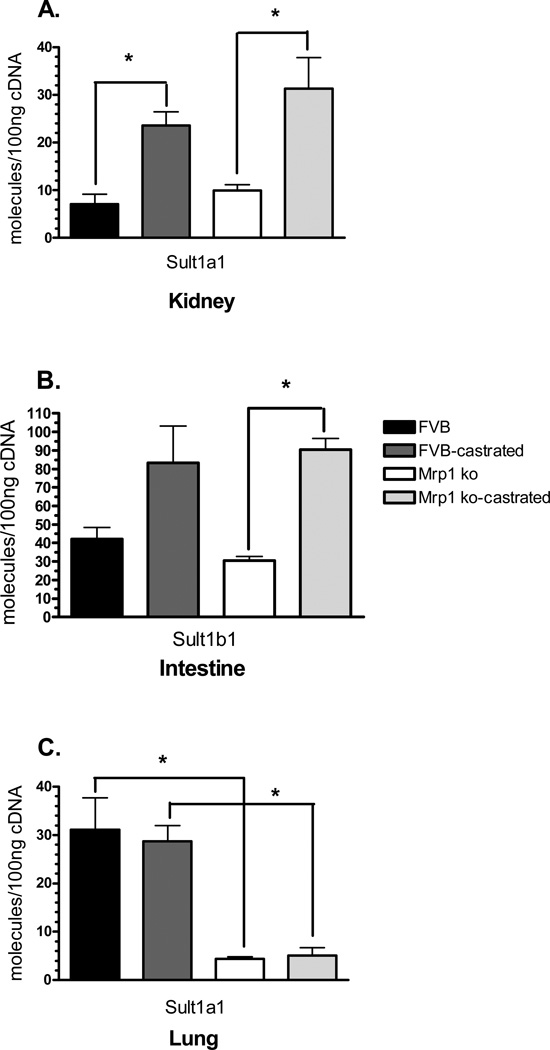
A very similar pattern of tissue regulation was found with Sult transcript levels, although Sult1a1 was expressed in the kidneys and lungs, while Sult1b1 was only expressed in the intestines. As with the Ugts in the kidney, Sult1a1 expression was dependent upon hormone status, being increased by ~3.3-fold in castrated mice (Fig. 3A). In the intestines, differences in Sult expression were only seen in mice lacking Mrp1, with expression being increased by 3-fold owing to the loss of testosterone (Fig. 3B). In the lungs, differences in Sult expression are due to genotype, with expression of Sult1a1 being decreased by 7.8-fold in the intact mice lacking Mrp1 and decreased by 5.6-fold in the castrated mice lacking Mrp1 (Fig. 3C), similar to that seen in Ugt expression.
Figure 3.
mRNA levels of Sults in the kidneys, lung, and small intestine. mRNA levels were determined by qPCR using primers specific for Sult1a1 or Sult1b1. Samples (n = 3) were run in triplicate and repeated twice. Data are expressed as copy number per 100 ng cDNA ± SD normalized to Gapdh. Statistical differences (*) were determined by ANOVA followed by Tukey’s test (P < 0.05). Sult1a1 is expressed in only in the kidney (A) and lung (C). Sult1b1 is only expressed in the intestine (B).
Phase III Transporter mRNA Expression in FVB and Mrp1−/− Mice
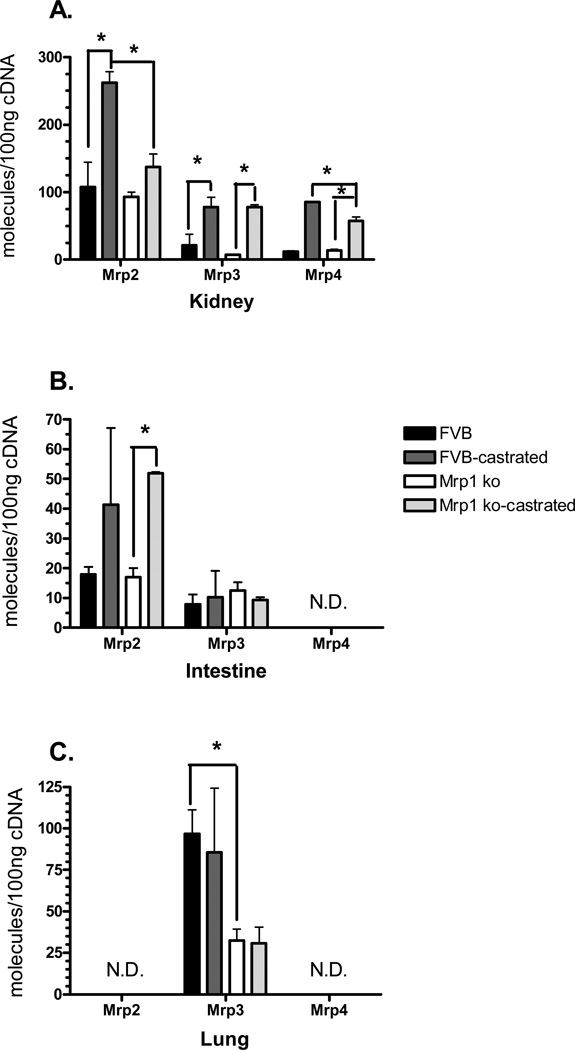
Transcript levels of Mrp2, 3 and 4 were next examined in the kidneys, intestines and lungs of mice, although Mrp4 expression was below the detection limit in the small intestines and lungs, while Mrp2 was not detected in the lung (Fig. 4). The patterns of transporter expression mirrored that of the phase II enzymes, as Mrp2, 3 and 4 transcripts increased in the kidneys of castrated mice, regardless of the genotype (Fig. 4A). In the small intestine, Mrp2 expression was increased by 3-fold, but only in the mice lacking Mrp1 (Fig. 4B). In the lungs, only the genotype resulted in changes, as Mrp3 was downregulated by 2.8-fold in the Mrp1 knockout mice as compared with the wildtype animals (Fig. 4C).
Figure 4.
Mrp gene expression in the kidneys, lung, and small intestine. mRNA levels in the kidney (A), small intestine (B) and lung (C) were determined by qPCR using primers specific for each Mrp family member. Samples (n = 3) were run in triplicate and repeated twice. Data are expressed as copy number per 100 ng cDNA ± SD normalized to Gapdh. Statistical differences (*) were determined by ANOVA followed by Tukey’s test (P < 0.05). ND, Not detected.
Nrf2 Expression
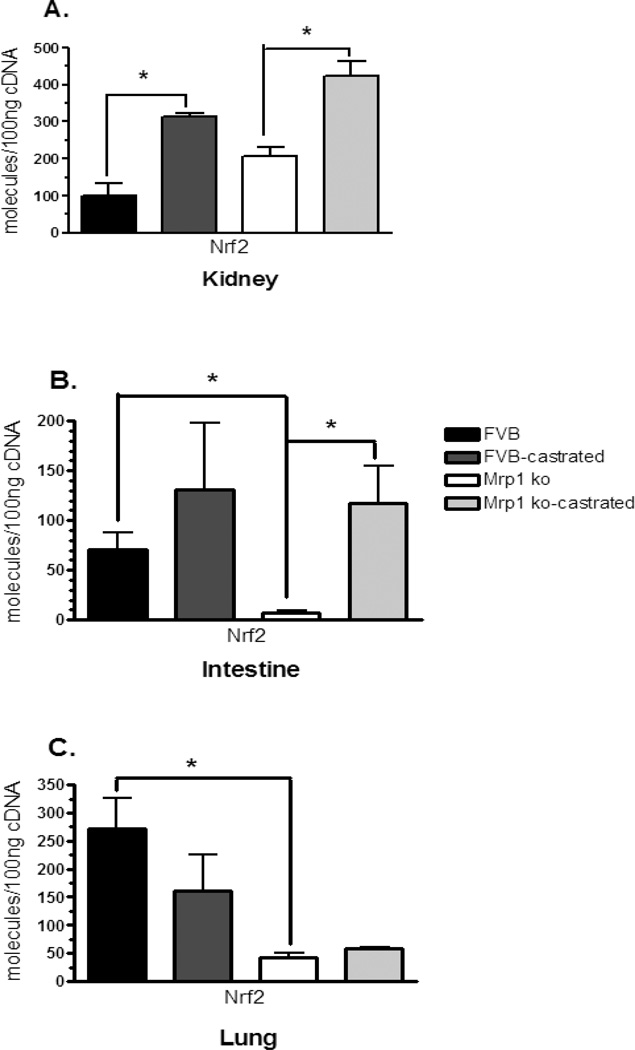
Expression of Mrp1 is predominantly regulated by the nuclear factor-E2 p45-related factor2 (Nrf2) (Hayashi et al., 2003), and the highest levels of Nrf2 transcript were seen in the lung, followed by both the small intestine and kidney (Itoh et al., 1997). We therefore examined whether changes in Nrf2 expression could be the mechanism responsible for the altered phase II and III enzyme levels. Indeed, Nrf2 expression was increased in the kidney by 2- to 3.1-fold when the mice were castrated, regardless of genotype (Fig. 5A). In the intestine, Nrf2 expression was increased by nearly 16-fold in the castrated Mrp1−/− compared with the intact mice. Interestingly, Nrf2 transcript was also reduced by nearly 10-fold in the Mrp1 knockout animals as compared with the wildtype mice (Fig. 5B). In the lungs, Nrf2 expression was decreased by 6.3-fold in the mice lacking Mrp1 compared with the FVB mice (Fig. 5C). Almost all of the patterns mimic the expression of the phase II and phase III enzymes in these animals (Table 2).
Figure 5.
Nrf2 transcript levels in the kidneys, lung, and small intestine. mRNA levels in the kidney (A), small intestine (B) and lung (C) were determined by qPCR using primers specific for Nrf2. Samples (n = 3) were run in triplicate and repeated twice. Data are expressed as copy number per 100 ng cDNA ± SD normalized to Gapdh. Statistical differences (*) were determined by ANOVA followed by Tukey’s test (P < 0.05).
Table 2.
Correlation between Nrf2 and tissue-specific expression patterns of phase II and phase III enzymes
| Kidney | Intestine | Lung | |
|---|---|---|---|
| A. Changes owing to castration | |||
| Nrf2 | ↑ | ↑ | — |
| Sult | ↑ | ↑ | — |
| Ugt1a | ↑ | ↑ | —/↑ |
| Ugt2b | ↓ | ↓ | — |
| Mrp | ↑ | ↑ | — |
| B. Changes owing to the loss of Mrp1 | |||
| Nrf2 | — | ↓ | ↓ |
| Sult | — | — | ↓ |
| Ugt1a | —/↓ | — | ↓ |
| Ugt2b | — | — | ↓ |
| Mrp | —/↓ | — | ↓ |
Discussion
This study indicated that the loss of Mrp1 coupled with reductions in steroid hormone levels leads to alterations in extrahepatic phase I, phase II and phase III metabolizing enzymes in a tissue-specific manner. Typically, studies involving compensatory responses after the loss of a transporter focus on examining the liver (Bain and Feldman, 2003; Kitamura et al., 2010; Nezasa et al., 2006), since this is the organ with the highest detoxification activity and readily eliminates compounds into the bile. However, since the baseline expression of Mrp1 in the liver is low (Roelofsen et al., 1997), extrahepatic tissues, such as the kidney, lungs and small intestine, are more likely to play a role in Mrp-1 mediated drug absorption and elimination.
Our data indicate that, in the lungs, the levels of Sults, Ugts and Mrps were reduced in mice lacking Mrp1 based upon genotype. Little data exist showing compensatory detoxification enzyme changes in the lungs. It is known that Mrp1 is predominantly regulated by the nuclear factor-E2 p45-related factor2 (Nrf2), which binds to antioxidant responsive elements in its promoter (Hayashi et al., 2003). Indeed, studies have shown that knockdown of Nrf2 decreases Mrp1 expression (Song et al., 2009). The highest expression levels of Nrf2 are in the lung, followed equally by the intestine and the kidney (Itoh et al., 1997). In the lungs, it is thought the Nrf2 is at such high levels to protect this tissue from oxidative stress (Cho et al., 2006).
Nrf2 regulates the expression of Mrp1, and also regulates the expression of other phase II and III enzymes, including Mrp2, 3 and 4 (Mahaffey et al., 2005b, 2008, 2009). While Nrf2 is at high levels in the lungs and may be the predominant regulator of detoxification enzyme expression in that tissue, in other tissues, different nuclear receptors may play a larger role in controlling phase II and III enzyme expression (Mahaffey et al., 2009; Maher et al., 2005b, 2008). For example, in mice lacking Nrf2, hepatic expression of Mrp2 and Mrp3 was reduced by 68 and 96%, respectively, as was the expression of CAR, PXR and AhR (Anwar-Mohamed et al., 2011). However, at least in our study, Mrp expression in the kidney and in the intestine typically followed Nrf2 expression.
As with the Mrp transporters, Nrf2 inducers have been shown to increase Sult1a1 and Sult1b1 expression in mouse livers (Alnouti and Klaassen, 2008). In the current study, expression of Sults also follows that of Nrf2. Similarly, Ugt1 expression is generally induced by Nrf2 (Yueh and Tukey, 2007). In contrast, Ugt2b expression is generally repressed by Nrf2, although this pattern of regulation is not strictly followed in the intestine, in which Ugts appear to be regulated more by AhR and CAR (Buckley and Klaassen, 2009b). Our results indicate that Ugt expression follows Nrf2 expression in most cases. In the kidney, Nrf2 expression is increased owing to castration and therefore Ugt1a expression increases and Ugt2b expression decreases owing to castration. In the lungs, Ugt1a transcript is low. Ugt2b transcript expression followed that of Nrf2, in that it was repressed in mice lacking Mrp1. In the small intestine, Nrf2 expression is more complex: it decreases based on genotype and increases owing to castration. Thus, Ugt1a expression is increased and Ugt2b expression is decreased in castrated animals. Nrf2 expression is reduced in the intestines of Mrp1 knockout animals. Although Ugt2b expression is increased by 2.5-fold between FVB and Mrp1−/− animals, this is not quite statistically significant (P = 0.07). Without Mrp1, Nrf2 levels are altered in a tissue-specific manner, leading to changes in phase II and III enzyme expression.
In addition to regulation by Nrf2, many of the phase I, II and III enzymes are regulated in a sex-predominant or sex-specific manner, either owing to the regulation by androgens and estrogens, or by sex-specific differences in growth hormone release (Alnouti and Klaassen, 2011; Buckley and Klaassen, 2009a; Maher et al., 2006; Waxman, 1984). Previous studies had shown that basal levels of serum and testicular testosterone were reduced by 2.4- to 5-fold in Mrp1 knockout mice (Sivils et al., 2010). Thus, we wanted to determine whether any compensatory responses were also due to changes in androgen levels, particularly in the kidney and in the small intestine.
There are a number of sex differences in detoxification enzymes. Although intestinal and lung Mrp levels have not been shown to be different, both Mrp3 and Mrp4 are more highly expressed in the kidneys of female mice (Maher et al., 2005a), which is thought to be due to an increase in estrogens coupled with the lack of androgens (Maher et al., 2006). Similarly in the present study, when male mice are castrated, regardless of genotype, Mrp3 and Mrp4 expression increases in the kidney. In the kidney, Ugts are also expressed in sex-predominant expression patterns. Ugt1a2 is female-predominant, while Ugt2b38 is male-predominant, both of which are due to the inductive or suppressive effects of testosterone (Buckley and Klaassen, 2009a). Again, this concurs with the present study in which Ugt expression in the kidney is changed by castration such that Ugt1 expression increases and Ugt2 expression decreases.
Sult1a appears to be regulated more so by androgens, estrogens and growth hormone than the other phase II or III enzymes. In the present study Sult1a1 expression was reduced in the lung, which may be related to increased serum estrogen levels (Sivils et al., 2010). Sult1a1 is the only Sult with higher expression in the male compared with female mice and this only occurs in the lung (Alnouti and Klaassen, 2006). Renal Sult1a1 expression is generally 2- to 3-fold higher in females than in males (Alnouti and Klaassen, 2006). Our study also indicates that castrated males, regardless of genotype, have increased Sult1a1 expression in the kidneys compared with the intact mice. There is a slight 1.4-fold increase in transcript levels in the Mrp1 knockout animals, albeit not significant, which may indicate that the reduction in serum testosterone in the knockouts is having some effect on Sult1a1 expression. The mechanisms behind the higher expression in females are thought to be controlled by both the lack of androgens and the pulsatile nature of growth hormone secretion in males (Alnouti and Klaassen, 2011). While Mrp1 knockout mice have 2.4-fold reduced circulating levels of testosterone compared with wildtype mice, androstenedione levels are equivalent between FVB and Mrp1 knockout (Sivils et al., 2010). Therefore, androgen concentrations may still be high enough in the Mrp1 knockouts to keep the more male-like expression of Sult1a1.
The link between Nrf2 expression and steroid hormone levels is not well understood, and most work has been conducted in breast or prostate tissue. It is known that the reactive catechol estrogens, such as 2-hydroxyestrogen and 4-hydroxyestrogen, can activate and upregulate Nrf2-mediated gene expression (Lee et al., 2003; Sumi et al., 2009). However, other investigators have shown that inhibiting estrogen-receptor signaling pathways activates Nrf2-mediated transcription, which would suggest that increased estrogen signaling leads to a reduction in Nrf2 levels (Yao et al., 2010). In the current study, Nrf2 expression increases in the kidney and intestine after castration, but appears to decrease in the lungs. So whether the altered estrogen levels in castrated animals directly activate Nrf2 leading to the changes in phase II and phase III enzymes, or whether Nrf2 expression is dependent on some other mechanism remains to be determined.
The loss or reduction of detoxification enzymes, such as Mrp1, is an important pharmacological response to many drugs and other xenobiotics, as it can alter drug disposition, toxicity and cellular reactive oxygen species. Although most work has been done in rodents, where knockouts are available, there is evidence that a reduction in human MRP1 is clinically relevant. For example, MRP1 extrudes glutathione during periods of oxidative stress, including apoptosis (Mueller et al., 2005; Widder et al., 2007). When MRP1 levels are reduced, glutathione efflux is dramatically reduced (Marchan et al., 2008), leading to reductions in intracellular ROS formation (Song et al., 2011) and making cells less susceptible to apoptosis (Marchan et al., 2008). Since Nrf2 regulates the expression of Mrp1 as well other phase II and III enzymes, it appears that this coordinate regulation is useful to protect cells from oxidative stress.
In conclusion, compensatory responses in extrahepatic tissues owing to the loss of Mrp1 vary depending on the tissue, probably owing to changes in both Nrf2 expression and hormonal regulation. This information is important to help predict how compensatory responses can alter xenobiotic toxicity, disposition and elimination in important, extrahepatic target tissues.
Acknowledgments
This study was supported by NIH (ES012417).
References
- Akita H, Suzuki H, Ito K, Kinoshitaa S, Satoa N, Takikawab H, Sugiyama Y. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim. Biophys. Acta. 2001;1511:7–16. doi: 10.1016/s0005-2736(00)00355-2. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol. Sci. 2006;93:242–255. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J. Pharmacol. Exp. Ther. 2008;324:612–621. doi: 10.1124/jpet.107.129650. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Mechanisms of gender-specific regulation of mouse sulfotransferases (Sults) Xenobiotica. 2011;41:187–197. doi: 10.3109/00498254.2010.535923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar-Mohamed A, Degenhardt OS, El Gendy MA, Seubert JM, Kleeberger SR, El-Kadi AO. The effect of Nrf2 knockout on the constitutive expression of drug metabolizing enzymes and transporters in C57Bl/6 mice livers. Toxicol. Vitro. 2011;25:785–795. doi: 10.1016/j.tiv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Bain LJ, Feldman RA. Altered expression of sulfotransferases, glucuronosyltransferases, and mrp transporters in FVB/mrp1−/− mice. Xenobiotica. 2003;33:1173–1183. doi: 10.1080/00498250310001609138. [DOI] [PubMed] [Google Scholar]
- Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pfluger's Arch. 2007;453:621–641. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, LeBlanc GA. The anti-carcinogenic plant compound indole-3-carbinol differentially modulates P450-mediated steroid hydroxylase activities in mice. Chem. Biol. Interact. 1992;83:155–169. doi: 10.1016/0009-2797(92)90043-k. [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD. Characterization of MOAT-C and MOAT-D new members of the MRP/cMOAT subfamily of transporter proteins. J. Natl Cancer Inst. 1998;90(22):1735–1741. doi: 10.1093/jnci/90.22.1735. [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab. Dispos. 2007;35:121–127. doi: 10.1124/dmd.106.012070. [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. Mechanism of gender-divergent UDP-glucuronosyltransferase mRNA expression in mouse liver and kidney. Drug Metab. Dispos. 2009a;37:834–840. doi: 10.1124/dmd.108.024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab. Dispos. 2009b;37:847–856. doi: 10.1124/dmd.108.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Slitt AL, Dieter MZ, Tanaka Y, Scheffer GL, Klaassen CD. Up-regulation of Mrp4 expression in kidney of Mrp2-deficient TR(−) rats. Biochem. Pharmacol. 2005;70:1088–1095. doi: 10.1016/j.bcp.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. Organ distribution of multidrug resistance proteins 1, 2, and 3 (mrp 1, 2, and 3) mRNA and hepatic induction of mrp3 by constitutive androstane receptor activators in rats. J. Pharmacol. Exp. Ther. 2002;300(1):97–104. doi: 10.1124/jpet.300.1.97. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Chu X, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, Evers R. Characterization of mice lacking the multidrug resistance protein Mrp2 (Abcc2) J. Pharmacol. Exp. Ther. 2006;317:579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1) FEBS Lett. 2006;580:1103–1111. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima T. An Nrf2/small maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Comm. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KL. Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab. Dispos. 2005;34:556–562. doi: 10.1124/dmd.105.005793. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Kusuhara H, Sugiyama Y. Functional characterization of multidrug resistance-associated protein 3 (Mrp3/Abcc3) in the basolateral efflux of glucuronide conjugates in the mouse small intestine. J. Pharmacol. Exp. Ther. 2010;332:659–666. doi: 10.1124/jpet.109.156943. [DOI] [PubMed] [Google Scholar]
- Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJT, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57(16):3537–3547. [PubMed] [Google Scholar]
- Kruh GD, Zeng H, Rea PA, Liu G, Chen Z-S, Lee K, Belinsky MG. MRP subfamily transporters and resistance to anticancer agents. J. Bioenerg. Biomembr. 2001;33(6):493–501. doi: 10.1023/a:1012827221844. [DOI] [PubMed] [Google Scholar]
- Lee JM, Anderson PC, Padgitt JK, Hanson JM, Waters CM, Johnson JA. Nrf2, not the estrogen receptor, mediates catechol estrogen-induced activation of the antioxidant responsive element. Biochim. Biophys. Acta. 2003;1629:92–101. doi: 10.1016/j.bbaexp.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lee K, Belinsky MG, Bell DW, Testa JR, Kruh GD. Isolation of MOAT-B, a widely expressed multidrug resistance-associated protein/canalicular multispecific organic anion transporter-related transporter. Cancer Res. 1998;58:2741–2747. [PubMed] [Google Scholar]
- Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57:5238–5242. [PubMed] [Google Scholar]
- Mahaffey CM, Zhang H, Rinna A, Holland W, Mack PC, Forman HJ. Multidrug-resistant protein-3 gene regulation by the transcription factor Nrf2 in human bronchial epithelial and non-small-cell lung carcinoma. Free Radic. Biol. Med. 2009;46:1650–1657. doi: 10.1016/j.freeradbiomed.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J, Aleksunes L, Dieter M, Tanaka Y, Peters J, Manautou J, Klaassen C. Nrf2 and PPAR{alpha}-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol. Sci. 2008 doi: 10.1093/toxsci/kfn177. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington N, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab. Dispos. 2005a;33:947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab. Dispos. 2005b;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem. Pharmacol. 2006;71:1470–1478. doi: 10.1016/j.bcp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Marchan R, Hammond CL, Ballatori N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim. Biophys. Acta. 2008;1778:2413–2420. doi: 10.1016/j.bbamem.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CF, Widder JD, McNally JS, McCann L, Jones DP, Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circul. Res. 2005;97:637–644. doi: 10.1161/01.RES.0000183734.21112.b7. [DOI] [PubMed] [Google Scholar]
- Nezasa K, Tian X, Zamek-Gliszczynski MJ, Patel NJ, Raub TJ, Brouwer KL. Altered hepatobiliary disposition of 5 (and 6)-carboxy-2',7'-dichlorofluorescein in Abcg2 (Bcrp1) and Abcc2 (Mrp2) knockout mice. Drug Metab. Dispos. 2006;34:718–723. doi: 10.1124/dmd.105.007922. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. Characterization of inducible nature of MRP3 in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G438–G446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- Peng K-C, Cluzeaud F, Bens M, Van Huyen JP, Wioland MA, Lacave R, Vandewalle A. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J. Histochem. Cytochem. 1999;47(6):757–768. doi: 10.1177/002215549904700605. [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Vos TA, Schippers IJ, Kuipers F, Koning H, Moshage H, Jansen PLM, Muller M. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells. Gastroenterology. 1997;112:511–521. doi: 10.1053/gast.1997.v112.pm9024305. [DOI] [PubMed] [Google Scholar]
- Sivils JC, Gonzalez I, Bain LJ. Mice lacking MRP1 have reduced testicular steroid hormone levels and alterations in steroid biosynthetic enzymes. Gen. Comp. Endocrinol. 2010;167:51–59. doi: 10.1016/j.ygcen.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song NY, Kim DH, Kim EH, Na HK, Surh YJ. 15-Deoxy-delta 12, 14-prostaglandin J2 induces upregulation of multidrug resistance-associated protein 1 via Nrf2 activation in human breast cancer cells. Ann. NY Acad. Sci. 2009;1171:210–216. doi: 10.1111/j.1749-6632.2009.04914.x. [DOI] [PubMed] [Google Scholar]
- Song NY, Kim DH, Kim EH, Na HK, Kim NJ, Suh YG, Surh YJ. Multidrug Resistance-associated protein 1 mediates 15-deoxy-Δ(12,14)-prostaglandin J(2)-induced expression of glutamate cysteine ligase expression via Nrf2 signaling in human breast cancer cells. Chem. Res. Toxicol. 2011;24:1231–1241. doi: 10.1021/tx200090n. [DOI] [PubMed] [Google Scholar]
- Stride BD, Valdimarsson G, Gerlach JH, Wilson GM, Cole SPC, Deeley RG. Structure and expression of the messenger RNA encoding the murine multidrug resistance protein, an ATP-binding cassette transporter. Mol. Pharmacol. 1996;49:962–971. [PubMed] [Google Scholar]
- Sumi D, Numasawa Y, Endo A, Iwamoto N, Kumagai Y. Catechol estrogens mediated activation of Nrf2 through covalent modification of its quinone metabolite to Keap1. J. Toxicol. Sci. 2009;34:627–635. doi: 10.2131/jts.34.627. [DOI] [PubMed] [Google Scholar]
- Toyoda Y, Hagiya Y, Adachi T, Hoshijima K, Kuo MT, Ishikawa T. MRP class of human ATP binding cassette (ABC) transporters: historical background and new research directions. Xenobiotica. 2008;38:833–862. doi: 10.1080/00498250701883514. [DOI] [PubMed] [Google Scholar]
- Waxman DJ. Rat hepatic cytochrome P-450 isoenzyme 2c. Identification as a male-specific, developmentally induced steroid16 alpha-hydroxylase and comparison to a female-specific cytochrome P-450 isoenzyme. J. Biol. Chem. 1984;259:15481–15490. [PubMed] [Google Scholar]
- Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- Xia CQ, Milton MN, Gan LS. Evaluation of drug–transporter interactions using in vitro and in vivo models. Curr. Drug Metab. 2007;8:341–363. doi: 10.2174/138920007780655423. [DOI] [PubMed] [Google Scholar]
- Yao Y, Brodie AM, Davidson NE, Kensler TW, Zhou Q. Inhibition of estrogen signaling activates the NRF2 pathway in breast cancer. Breast Cancer Res. Treat. 2010;124:585–591. doi: 10.1007/s10549-010-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh M-F, Tukey RH. Nrf2-Keap1 signalling pathway regulates human UGT1A1 expression in vitro and in transgenic UGT1 mice. J. Biol. Chem. 2007;282:8749–8758. doi: 10.1074/jbc.M610790200. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]