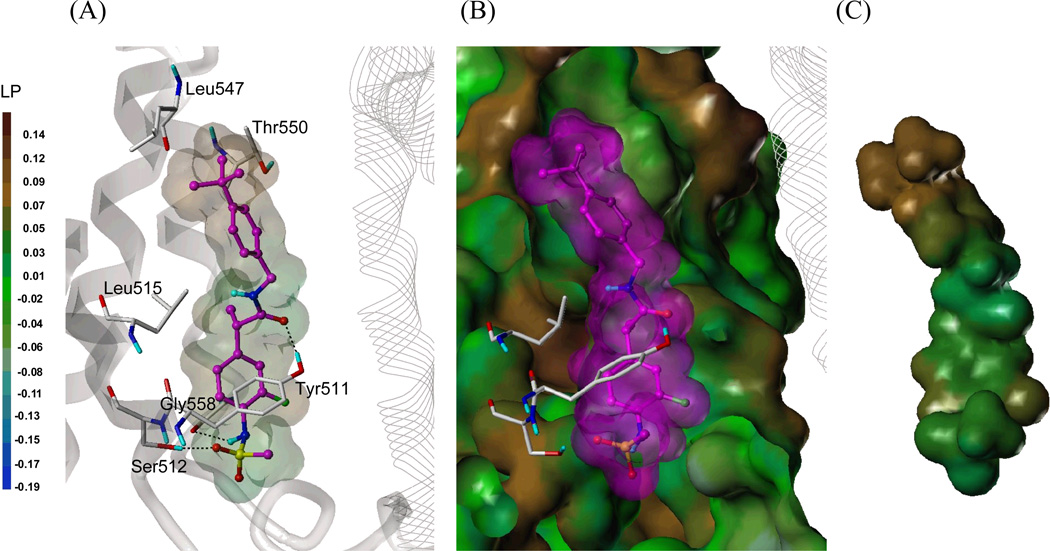
Figure 2. Predicted binding mode of 54S in hTRPV1 homology model with surface representations.
(A) Docked mode of 54S. The key interacting residues are marked and displayed as a capped-stick representation with carbon atoms in white. The helices are colored in gray and the helices of the neighboring monomer are displayed in a line ribbon representation. The ligand is depicted as a ball-and-stick with carbon atoms in magenta and its van der Waals surface is presented with lipophilic potential property (LP) which ranges from brown (highest lipophilic area) to blue (highest hydrophilic area). Hydrogen bonds are drawn in black dashed lines, and non-polar hydrogens are undisplayed for clarity. (B) Surface representations of the docked 54S and hTRPV1. The Fast Connolly surface of hTRPV1 was generated by MOLCAD and colored by the lipophilic potential. For clarity, the surface of hTRPV1 is Z-clipped and that of the ligand is in its carbon color. (C) Van der Waals surface of 54S colored by the lipophilic potential.