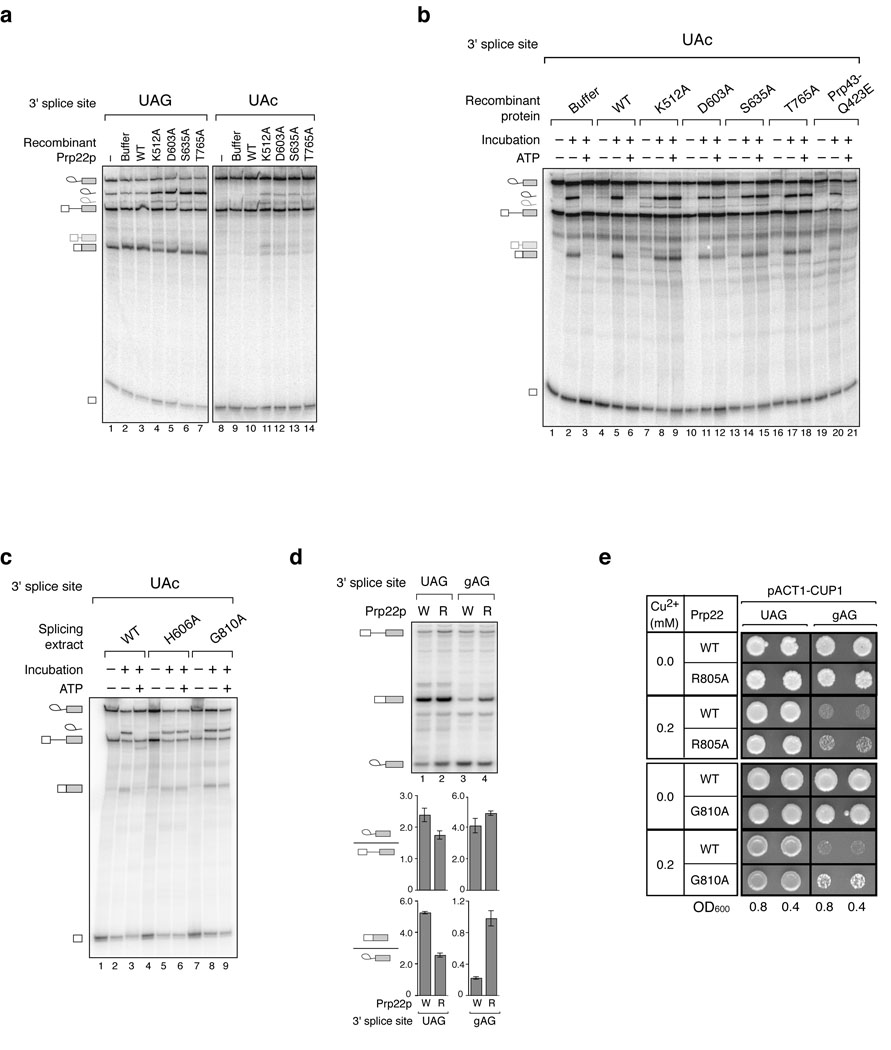
Figure 3.
Repression of aberrant exon ligation at mutated 3’ splice sites is compromised in vitro and in vivo by ATPase- and RNA unwindase-deficient variants of the DEAH-box ATPase Prp22p. (a,b) Recombinant Prp22p variants30,42–44 abolish repression of an aberrant 3’ splice site in extract (a) or in affinity-purified spliceosomes (b). Wild-type (UAG) or mutated (UAc) pre-mRNA was incubated in wild-type extract supplemented with nothing (−), buffer, wild-type Prp22p (WT) or mutated Prp22p (amino acid mutations are indicated) in standard splicing reactions. In a, these reactions were analyzed directly; in b, the spliceosomes stalled in these reactions were affinity purified and then treated as in Figure 1b. In b, a Prp43p variant was analyzed in parallel. Note that Prp22p variants block dissociation and subsequent degradation of the excised intron (e.g., ref. 42). Migration of cryptic 3’ splice site cleavage products (see text) are indicated to the left as light gray excised intron and mRNA symbols. (c) Endogenous, Prp22p variants also abolish repression of an aberrant 3’ splice site in purified spliceosomes. Mutated (UAc) pre-mRNA was incubated in reactions with extract of a wild-type PRP22 (WT) strain and the cold-sensitive mutant strains prp22-H606A and prp22-G810A (ref. 42). Spliceosomes stalled in these reactions were immunoprecipitated and then treated as in Figure 1b. The faster migration of excised lariat intron in this gel is due to the concentration of polyacrylamide. (d) A prp22 mutant is defective in discriminating between a wild-type and a mutated 3’ splice site in vivo. A wild-type PRP22 (W) or the mutant prp22-R805A (R) strain42 was transformed with an ACT1-CUP1 splicing reporter48 having a wild-type UAG or mutated gAG 3’ splice site. Splicing was analyzed by primer extension. The ratio of mRNA to lariat intermediate is plotted as an indicator of the apparent efficiency of exon ligation; the ratio of lariat intermediate to pre-mRNA is plotted for comparison. The averages and range of values for 2 independent experiments are shown. (e) prp22 mutants are defective in repressing gene expression from an ACT1-CUP1 splicing reporter48 having the mutated gAG 3’ splice site. A wild-type PRP22 (WT) strain and the mutant strains prp22-R805A (R805A) and prp22-G810A (G810A) were transformed with an ACT1-CUP1 reporter having a wild-type (UAG) or mutated (gAG) 3’ splice site. Cells diluted to 0.8 or 0.4 OD600 were spotted onto plates containing 0.0 or 0.2 mM CuSO4 and grown for 3 days at 33 °C (R805A) or 30 °C (G810A).